Three Treatments for Osteoarthritis of the Knee: Evidence Shows Lack of Benefit
Clinician Summary Guide published 8 Apr 2009
Table of Contents
- Introduction
- Clinical Bottom Line
- Glucosamine and Chondroitin
- Viscosupplementation
- Arthroscopic Surgery for Osteoarthritis
- Other Options
- Resources for Patients
- Source
- For More Information
1. Introduction
This guide summarizes evidence on the effectiveness and safety of three treatments for osteoarthritis of the knee: use of the supplements glucosamine, chondroitin, or both combined; viscosupplementation (injection of hyaluronan into the knee); and arthroscopic lavage and debridement of the knee joint. The evidence evaluated comes primarily from comparisons of each treatment approach with a placebo. This guide does not address other treatments, such as exercise, physical therapy, pain medications, corticosteroid injections, or knee replacement.
Clinical Issue
Osteoarthritis (OA) is the most common form of chronic arthritis worldwide and is a key cause of pain and disability in older adults. In the United States, clinically significant disease affects 10-20 percent of individuals age 60 and over. Osteoarthritis of the knee, about twice as common as OA of the hip, is becoming an increasingly important condition with the aging of the U.S. population.
Osteoarthritis risk factors include injury, prior joint inflammation, abnormalities of joint shape, and obesity. The natural history of knee OA and the factors leading to its progression are not fully understood.
The goals of treatment are to reduce pain and improve joint function. Nonsteroidal anti-inflammatory drugs (NSAIDs) are commonly used as first-line treatment for OA pain, along with exercise and weight loss. The treatments described in this guide are intended to promote healing of damaged cartilage in the knee or to augment the composition of synovial fluid.
2. Clinical Bottom Line
Clinical Bottom Line
For people with osteoarthritis of the knee, the following treatments do not lead to clinically meaningful improvement.

Confidence Scale
The confidence ratings in this guide are derived from a systematic review of the literature. The level of confidence is based on the overall quantity and quality of clinical evidence.
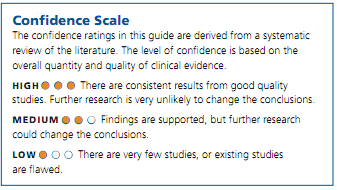
There are very few studies, or existing studies are flawed.
3. Glucosamine and Chondroitin
The dietary supplements glucosamine and chondroitin have been advocated as treatments for osteoarthritis pain based on the theory that they may increase the rate of new cartilage formation. Glucosamine is a precursor to glycosaminoglycan, which is believed to play a role in the growth of cartilage and its repair. Chondroitin is part of a large proteoglycan molecule that gives cartilage flexibility and is thought to inhibit enzymes that break down cartilage.
Both substances are found naturally, and supplements are made from animal tissue. Chondroitin sulfate is purified from animal cartilage, such as cartilage from cows or sharks. Glucosamine comes from the shells of crabs, lobsters, or shrimp.
They are both sold over the counter as oral supplements. Supplements are not subject to review or approval by the U.S. Food and Drug Administration and are not standardized. As such, the amount of active ingredients or possible contaminants may vary among products. Glucosamine is available in different forms, most commonly glucosamine sulfate or glucosamine hydrochloride.
Overall Treatment Benefit Not Seen
The best evidence on treatment efficacy of glucosamine and chondroitin comes from the Glucosamine/Chondroitin Arthritis Intervention Trial (GAIT). The GAIT study was a randomized controlled trial comparing groups of people receiving one of five treatments: glucosamine hydrochloride, chondroitin sulfate, the combination of both, celecoxib (an NSAID), or placebo. A total of 1,583 patients with OA of the knee were entered into the study.
In this study, improvement in global pain and joint function was observed in all groups – glucosamine, chondroitin, glucosamine and chondroitin combined, and placebo. However, there was no clinically significant effect on knee symptoms due to glucosamine and/or chondroitin supplementation. The trial also analyzed a subgroup of people with moderate to severe OA and found that glucosamine and chondroitin improved pain and joint function better than placebo treatment for this subgroup.
- Overall, people with OA of the knee do not have better relief of symptoms when using glucosamine and chondroitin than when receiving a placebo.

Adverse Effects
Some people taking glucosamine and chondroitin have experienced minor side effects, including nausea, diarrhea, and headache. However, there is no difference in the frequency of adverse effects among people using these supplements compared with those receiving placebo.
Still Unknown
While the salts of glucosamine contain similar components, evidence is insufficient to determine whether the effects of glucosamine sulfate differ from those of glucosamine hydrochloride.
Glucosamine is an amino sugar, but evidence is insufficient to determine whether its use leads to changes in glucose metabolism or glycemic control.
4. Viscosupplementation
Synovial fluid serves as a shock absorber to reduce friction from joint motion. Hyaluronic acid is a naturally occurring substance found in synovial fluid. As cartilage wears down in osteoarthritis, the level of hyaluronic acid in the joint also decreases.
The intra-articular injection of hyaluronan, known as viscosupplementation, is believed to improve the elastoviscosity of the arthritic joint by increasing the hyaluronic acid concentration. In clinical practice, hyaluronan injections are performed in an effort to reduce pain and improve function. The clinician enters the joint space with a needle, injecting a small amount of hyaluronan product (2.5- 5 cc of solution). The procedure is repeated from three to five times over a period of several weeks.
Treatment Benefit Not Seen
In people with osteoarthritis of the knee, published clinical trials comparing injections of viscosupplements with placebo have yielded inconsistent results. Higher quality and larger trials have generally found lower levels of clinical improvement in pain and function than small and poor quality trials.
- Any clinical improvement attributable to viscosupplementation is likely small and not clinically meaningful.

Various hyaluronan products are available for knee joint injection, primarily differing in the molecular weight of the compound. While some trials suggest better clinical response to the highest molecular weight hyaluronan product, other trials have not confirmed this finding.
Overall, evidence is insufficient to demonstrate clinical benefit for the higher molecular weight products.
Adverse Effects
Side effects from injection of hyaluronan products usually are minor and short term. Reported rates vary. Minor side effects include pain at the injection site (1-33 percent), local joint pain and swelling (< 1-30 percent), and local skin reactions (3-21 percent).
More serious side effects can occur. Pseudoseptic reactions, inflammation and swelling of the joint not caused by infection, are uncommon (1-3 percent) but can be severe and require further medical treatment.
Evidence is insufficient to determine whether the frequency of adverse events is higher with repeat injections.
5. Arthroscopic Surgery for Osteoarthritis
Arthroscopic surgery allows a surgeon to visualize the interior joint space. The procedure provides access to the joint for lavage, using saline irrigation to remove particulate material, such as cartilage fragments and calcium crystals. Arthroscopy also allows for debridement, whereby surgical instruments are used to smooth any rough articular surfaces. The goals of arthroscopic lavage and debridement are to decrease synovitis and improve joint motion.
Treatment Benefit Not Seen
Arthroscopic lavage, with or without debridement, does not improve pain and function for people with OA of the knee.
Adverse Effects
Side effects from arthroscopic lavage or lavage with debridement can include local pain and swelling, infection, prolonged drainage from the surgical site, bleeding into the joint, and thrombophlebitis.
6. Other Options
The available research has found none of the treatments reviewed in this guide to be effective for the general population of people with knee osteoarthritis. Thus, clinicians may need to consider other treatments.
AHRQ has released a separate guide for clinicians on non-narcotic pain medications for osteoarthritis. Analgesic medicines provide pain relief and may allow people to improve their activity levels. Other approaches, such as exercise and weight loss, can relieve knee symptoms and offer other health benefits as well.
For some people with severe osteoarthritis, total knee replacement may relieve symptoms and improve function. It is important to consider the potential risks and benefits of this surgical procedure. AHRQ has published an evidence report on total knee replacement that is available on the AHRQ Web site: www.ahrq.gov/clinic/tp/kneetp.htm.
7. Resources for Patients
Osteoarthritis of the Knee: A Guide for Adults is a companion to this Clinician’s Guide. It can help people develop strategies for managing knee osteoarthritis. It provides information about glucosamine/chondroitin, viscosupplementation, and arthroscopic surgery, as well as general self-help approaches.
Choosing Pain Medicine for Osteoarthritis: A Guide for Consumers provides information about both prescription and over-the-counter non-narcotic analgesic medications, including (NSAIDs).
8. Source
The source material for this guide is a systematic review of 86 research publications. The review, Treatment of Primary and Secondary Osteoarthritis of the Knee (2008), was prepared by Blue Cross and Blue Shield Association Technology Evaluation Center Evidence-based Practice Center. The Agency for Healthcare Research and Quality (AHRQ) funded the systematic review and this guide. The guide was developed using feedback from clinicians who reviewed preliminary drafts.
AHRQ created the John M. Eisenberg Center at Oregon Health & Science University to make research useful for decisionmakers. This guide was written by Susan Woods, M.D., Erin Davis, B.A., Martha Schechtel, R.N., Valerie King, M.D., and David Hickam, M.D., of the Eisenberg Center.
9. For More Information
For free print copies, call:
The AHRQ Publications Clearinghouse
(800) 358-9295.
Consumer’s Guide, AHRQ Pub. No. 09-EHC001-A
Clinician’s Guide, AHRQ Pub. No. 09-EHC001-3





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 