學名:mycophenolic acid
作用為腎臟移植時的免疫抑制劑,預防器官的排斥作用。
機轉:
- Mycophenolic sodium is a reversible and uncompetitive inhibitor of inosine monophosphate dehydrogenase thereby inhibiting the de novo synthesis pathway of the guanosine nucleotide without incorporation into DNA. It exerts a potent cytostatic effect on B and T lymphocytes by inhibiting de novo synthesis because it is a crucial pathway for proliferation .
給藥劑量:
- negative serum or urine pregnancy test (sensitivity of at least 25 mIU/mL) within 1 week prior to initiation in all women of childbearing age required; do not start until a negative pregnancy test is reported; 2 forms of contraceptives should be used 4 weeks prior to starting mycophenolate, unless abstinence is the chosen method; continue contraceptives for 6 weeks after stopping therapy
- Renal transplant rejection; Prophylaxis: 720 mg ORALLY twice daily on an empty stomach
錠劑長相:
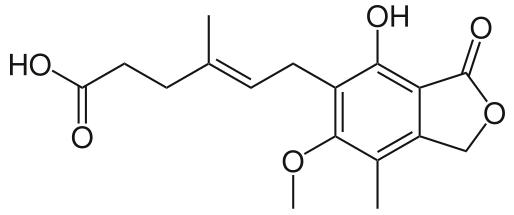
結構:
目前FDA有提出一則警告:
FDA通知醫護人員,藥物安全委員會發現預防器官排斥現象的免疫抑制劑Cellcept和Myfortic與病患罹患漸進性多病灶腦白質病
(Progressive multifocal leukoencephalopathy,
PML)可能有關聯性。PML會影響病人的中樞神經系統是一種罕見且致命性的疾病,常發生於因疾病或使用藥物而產生免疫抑制現象的患者。FDA正在審查
Roche藥廠所提出的資料,包括因使用Cellcept或Myfortic而罹患PML的上市後不良反應報告,和Cellcept所提出的仿單修正資
訊。FDA要求Myfortic的製造藥廠Novartis提出此藥發生PML的不良反應數據,並要求修正其仿單,修正內容需包含Cellcept仿單資
訊中有關PML之相同資訊。FDA預估需要花兩個月的時間才能將上述之上市報告與仿單資訊審查完畢。當審查作業完成後,FDA會將結論與建議公告大眾。
在得知進一步資訊之前,病人使用免疫抑制劑,包含Cellcept或Myfortic時若發生局部性的神經異常的徵兆和症狀時,病患與醫護人員應特別警覺,因為可能與PML有關。
FDA informed healthcare professionals that the Agency is investigating
a potential association between the use of CellCept and Myfortic,
medicines used to prevent organ rejection, and the development of
progressive multifocal leukoencephalopathy (PML), a life-threatening
disease. PML is a rare disorder that affects the central nervous system
usually occurring in patients with immune systems suppressed by disease
or medicines. FDA is reviewing data submitted by Roche, including
postmarketing reports it has received of PML in patients who took
CellCept or Myfortic, and the proposed revisions to the CellCept
prescribing information. FDA has asked Novartis, the maker of Myfortic,
for data on PML cases and to revise the Myfortic prescribing
information to include the same information about PML included in the
CellCept prescribing information. FDA anticipates it may take about 2
months to complete its review of the postmarketing reports and the
proposed revisions to the prescribing information. As soon as the
review is completed, FDA will communicate the conclusions and
recommendations to the public.
Until further information is available, patients and healthcare
professionals should be aware of the possibility of PML, such as
localized neurologic signs and symptoms in the setting of a suppressed
immune system, including during therapy with CellCept and Myfortic.
針對懷孕的部份,仿單上面有描述:
- To avoid pregnancy, recommend patient start using at least two different reliable methods of contraception at the same time 4 weeks prior to drug therapy, during therapy, and at least 6 weeks after completion of therapy .
藥物動力學資料:
Absorption
- Oral: time to peak concentration, 1.5 h to 2.75 h
- Bioavailability: 72%
- Effect of food: decrease in Cmax by 33% and delay Tmax by 5 h (range 9 h to 20 h)
- Vd: 54 L +/- 25 L
- Protein binding: greater than 98%
- Glucuronyl transferase
- Metabolites: mycophenolic acid glucuronide (MPAG) and acyl glucuronide
- Renal: less than 60% as MPAG, 3% unchanged
- 8 h to 16 h





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 