最近減肥藥的新聞真多呀
Audience: Consumers, Pharmacy healthcare professionals
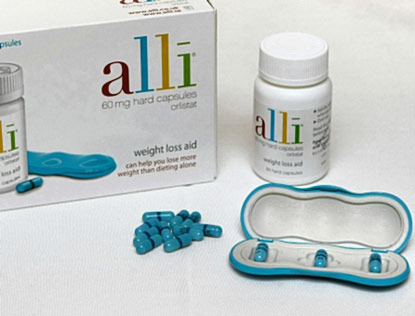
[Posted 01/18/2010] FDA notified consumers and healthcare professionals about a counterfeit and potentially harmful version of Alli 60 mg capsules (120 count refill kit). The counterfeit version contained the controlled substance sibutramine and did not contain orlistat, the active ingredient. Sibutramine is a drug that should not be used in certain patient populations or without physician oversight. Sibutramine can also interact in a harmful way with other medications the consumer may be taking. GSK has determined that the counterfeit product has been sold over the internet. However, there is no evidence at this time that the counterfeit Alli product has been sold through other channels, such as retail stores. The differences between the counterfeit and authentic products are described in both text and photos in the FDA news release.
Consumers who believe they have received counterfeit Alli are asked to contact the FDA's Office of Criminal Investigations (OCI) by calling 800-551-3989 or by visiting the OCI Web site (http://www.fda.gov/OCI).
Any adverse events that may be related to use should be reported to the FDA's MedWatch Safety Information and Adverse Event Reporting Program online [atwww.fda.gov/MedWatch/report.htm], by phone 1-800-332-1088, or by returning the postage-paid FDA form 3500 [which may be downloaded from the MedWatch "Download Forms" page] by mail [to address on the pre-addressed form] or fax [1-800-FDA-0178].
[01/18/2010 - News Release With Photos - FDA]





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 