
Purpose: The management of the drug interaction between atazanavir and tacrolimus in a renal transplant recipient is described.
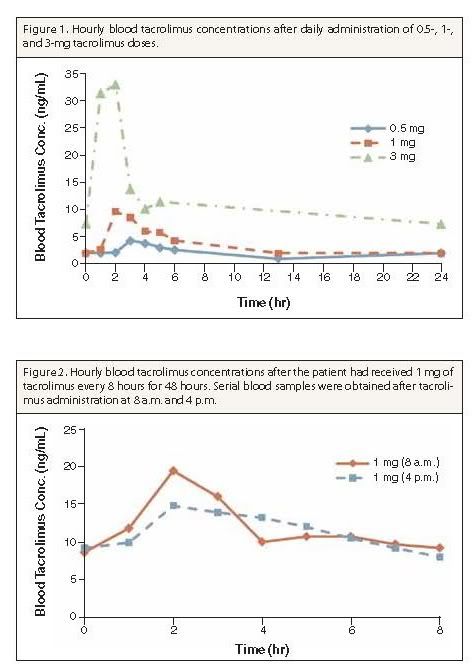
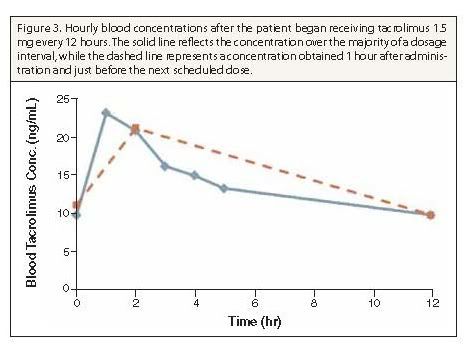
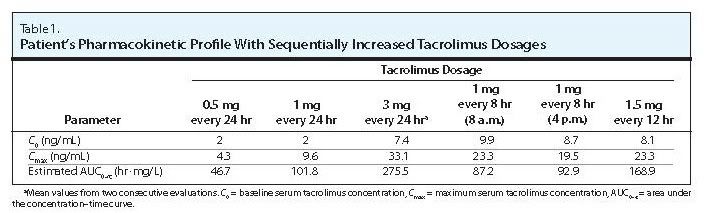
Summary: A 53-year-old African-American man with human immunodeficiency virus (HIV) received a renal transplant and was treated in accordance with a corticosteroid-sparing immunosuppressive protocol and maintenance immunosuppression with mycophenolate mofetil and tacrolimus. His highly active antiretroviral therapy included atazanavir 400 mg daily, abacavir 600 mg daily, and lamivudine 100 mg daily. Because of the potential for a significant interaction between tacrolimus and atazanavir, the tacrolimus dosage was to be based on serum tacrolimus concentrations. The patient was initially administered one dose of tacrolimus 0.5 mg on the morning of postoperative day 2. Evaluation of the tacrolimus profiles revealed that a higher dosage was necessary because serum tacrolimus levels decreased to subtherapeutic levels by 6 hours after dose administration. In an attempt to minimize tacrolimus toxicity and limit the duration of a subtherapeutic tacrolimus level, dosing was adjusted to 1 mg every 8 hours. After 48 hours of this regimen, peak serum tacrolimus levels were lower, and the drug concentrations remained at a relatively steady level throughout the dosing interval. One final dosage adjustment (1.5 mg every 12 hours) was performed to optimize serum tacrolimus levels and patient compliance.
Conclusion: In a 53-year-old man with HIV infection who underwent renal transplantation, the drug interaction between atazanavir and tacrolimus was managed by modifying the tacrolimus dosage regimen after determining the patient's blood tacrolimus concentration profile.





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 