Abstract and Introduction
Abstract
Objective: To update the evidence-based position statement published by The North American Menopause Society (NAMS) in 2006 regarding the management of osteoporosis in postmenopausal women.
Methods: NAMS followed the general principles established for evidence-based guidelines to create this updated document. A panel of clinicians and researchers expert in the field of metabolic bone diseases and/or women's health was enlisted to review the 2006 NAMS position statement, compile supporting statements, and reach consensus on recommendations. The panel's recommendations were reviewed and approved by the NAMS Board of Trustees.
Results: Osteoporosis, which is especially prevalent among older postmenopausal women, increases the risk of fractures. Hip and spine fractures are associated with particularly high morbidity and mortality in this population. Given the health implications of osteoporotic fractures, the primary goal of osteoporosis therapy is to prevent fractures, which is accomplished by slowing or stopping bone loss, maintaining bone strength, and minimizing or eliminating factors that may contribute to fractures. The evaluation of postmenopausal women for osteoporosis risk requires a medical history, physical examination, and diagnostic tests. Major risk factors for postmenopausal osteoporosis (as defined by bone mineral density) include advanced age, genetics, lifestyle factors (such as low calcium and vitamin D intake, smoking), thinness, and menopause status. The most common risk factors for osteoporotic fracture are advanced age, low bone mineral density, and previous fracture as an adult. Management focuses first on nonpharmacologic measures, such as a balanced diet, adequate calcium and vitamin D intake, adequate exercise, smoking cessation, avoidance of excessive alcohol intake, and fall prevention. If pharmacologic therapy is indicated, government-approved options are bisphosphonates, selective estrogen-receptor modulators, parathyroid hormone, estrogens, and calcitonin.
Conclusions: Management strategies for postmenopausal women involve identifying those at risk for fracture, followed by instituting measures that focus on reducing modifiable risk factors through dietary and lifestyle changes and, if indicated, pharmacologic therapy.
Introduction
Osteoporosis becomes a serious health threat for aging postmenopausal women by predisposing them to an increased risk of fracture. Osteoporotic fractures are associated with substantial morbidity and mortality in postmenopausal women, especially older women.
In response to the need to define standards of clinical practice in North America as they relate to menopause-associated health conditions, The North American Menopause Society (NAMS) has created this evidence-based position statement. The objective of this position statement is to provide guidance on the prevention, diagnosis, and treatment of osteoporosis in postmenopausal women to physicians, physician assistants, nurse practitioners, nurses, and other healthcare professionals caring for postmenopausal women, especially those in the clinical practice fields of obstetrics and gynecology, internal medicine, family medicine, and geriatrics.
This position statement is an update of the NAMS position statement published in 2006.[1] Since then, the publication of additional scientific evidence has created a need to update the position statement.
For this revision, NAMS conducted a search of the medical literature published since the previous position statement was submitted for publication in February 2006. A search was made for clinical trials, meta-analyses, and clinical practice guidelines published in English and related to osteoporosis in postmenopausal women, using the MEDLINE database. The Medical Subject Headings (MeSH) used for the search were postmenopausal osteoporosis and bone loss with subheadings for epidemiology, etiology, diagnosis, prevention and control, and therapy. The National Guideline Clearinghouse was searched for relevant clinical practice guidelines, and the Cochrane Library was searched for relevant systematic reviews. Priority was given to evidence from randomized controlled clinical trials and meta-analyses of such trials, followed by evidence from controlled observational studies, using criteria described elsewhere.[2–4] Conclusions from other evidence-based guidelines also were reviewed. Because standards of care and available treatment options differ throughout the world, the focus is limited to therapies available in North America.
To help with this revision, NAMS enlisted a five-person Editorial Board composed of endocrinologists, internists, and rheumatologists from both clinical practice and research with expertise in metabolic bone diseases or women's health. The Editorial Board reviewed the previous position statement and incorporated data published since that statement, compiled supporting statements, and made recommendations. Where the evidence was contradictory or inadequate to form a conclusion, a consensus-based opinion was established. (Practice parameter standards related to NAMS position statements have been described in an editorial.[5]) The NAMS Board of Trustees was responsible for the final review and approval of this document. Updates to this revised position statement will be published as developments occur in scientific research that substantially alters the conclusions.
Background
Osteoporosis—the most common bone disorder affecting humans—is a skeletal disorder characterized by compromised bone strength, predisposing a person to an increased risk of fracture.[6] Bone strength (and, hence, fracture risk) is dependent on many qualities of bone, of which bone mineral density (BMD) is the most commonly measured.[6] Expressed as grams of mineral per area or volume, BMD at any given age is a function of both peak bone mass (reached by age 30) and how much bone is subsequently lost. Qualities of bone other than BMD (including degree of mineralization, hydroxyapatite crystal size, collagen structure, heterogeneity of bone microstructure, connectivity of trabeculae, and microdamage) are difficult or impossible to measure in clinical practice at this time, although promising research is proceeding.
To standardize values from different bone densitometry tests, results are reported as either a Z-score or a T-score, with both expressed as standard deviation (SD) units.
- A T-score is useful to express BMD in a postmenopausal population and is calculated by comparing current BMD to the mean peak BMD of a normal, young adult population of the same gender. The reference database is white (non–race-adjusted) women, although this approach is not universally agreed upon.
- For premenopausal women under age 50, use of Z-scores is the preferred manner of expressing BMD.
- A Z-score is based on the difference between the person's BMD and the mean BMD of a reference population of the same gender, age, and ethnicity.
NAMS supports the World Health Organization (WHO) and International Society for Clinical Densitometry definitions[7] of osteoporosis in a postmenopausal woman or a man over age 50 as a BMD T-score less than or equal to −2.5 at the total hip, femoral neck, or lumbar spine (at least two vertebral levels measured in the posterior-anterior projection, not the lateral projection) (see Sidebar). If anatomic factors such as obesity or arthritis make measurements invalid, the distal one-third radius bone density may be considered a diagnostic site. However, the relationship between the T-score at this site and fracture risk has not been systematically examined.
| BMD-based definitions of bone density | |
| Normal: | T-score above (ie, better than) or equal to −1.0 |
| Low bone mass: a | T-score between −1.0 and −2.5 |
| Osteoporosis: | T-score below (ie, worse than) or equal to −2.5 |
| a Osteopenia From the World Health Organization.[7] |
|
In addition to diagnosis through densitometry, osteoporosis can be diagnosed clinically, regardless of the T-score. The presence of a fragility fracture constitutes a clinical diagnosis of osteoporosis.
Peak bone mass is achieved by a woman's third decade of life.[8] The process of bone loss begins at that time and accelerates at menopause. By age 80, many women have lost, on average, approximately 30% of their peak bone mass.[9]However, osteoporosis is not always the result of bone loss. A woman who does not achieve an adequate peak bone mass as a young adult may have low bone density without substantial bone loss as she ages.
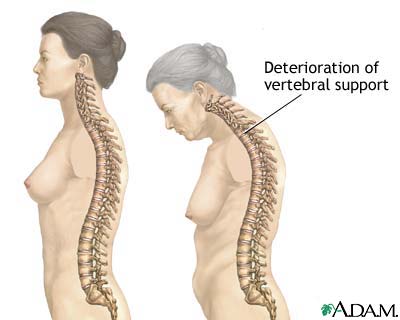
Osteoporosis has no warning signs. Often, the first indication of the disease is a fracture. Nearly all nonvertebral fractures are caused by a fall; however, vertebral fractures often occur without a fall, and need not necessarily be painful. Only roughly one third of vertebral fractures are painful, and two thirds are painless. Marked height loss over the years may be a sign of underlying vertebral compression fractures, even without significant associated back pain. Wrist or other fractures may occur at a younger age than vertebral or hip fractures and may also be early clinical expressions of osteoporosis.[10]
Osteoporosis is categorized as either primary or secondary. Primary osteoporosis is usually due to bone loss that occurs with aging. Secondary osteoporosis is a result of medications (eg, glucocorticoids) or diseases (eg, malabsorption) that adversely affect skeletal health.
The primary clinical goal of osteoporosis management is to reduce fracture risk. This may be accomplished by slowing or stopping bone loss, increasing bone mass or improving bone architecture, maintaining or increasing bone strength, and minimizing factors that contribute to falls. Management strategies include general preventive health measures and pharmacologic interventions.
Prevalence
Most cases of osteoporosis occur in postmenopausal women, and the prevalence of the disorder as defined by low BMD increases with age. Data from the Third National Health and Nutrition Examination Survey[11] indicate that 13% to 18% of white American women age 50 or older have osteoporosis of the hip, which the survey defined as femoral BMD at least 2.5 SD below the mean of young, healthy white women (ie, T-score of −2.5 or below). Another 37% to 50% have low bone mass (or osteopenia) of the hip, defined as a T-score between 1 and 2.5 SD below the mean.[11] The prevalence of osteoporosis rises from 4% in women ages 50 to 59 to 52% in women age 80 and older.[9]
Osteoporosis as defined by low BMD is a common contributor to fractures. Osteoporosis is responsible for an estimated 90% of all hip and spine fractures in white American women ages 65 to 84.[12] However, most postmenopausal women with fractures do not have bone density values consistent with osteoporosis, based on the WHO criterion.[13] In the Study of Osteoporotic Fractures,[14] 28% of hip fractures, 25% of vertebral fractures, and 13% of all fractures occurred in women with osteoporosis (total hip BMD of −2.5 or less). BMDs of −1.5 or lower were present in 51% of hip fracture subjects, 38% of vertebral fracture subjects, and 25% of all fracture subjects. In a 2-year follow-up of women older than age 65, 49% of hip fractures occurred in women with total hip BMD T-scores above −2.5; 28% occurred in women with T-scores above −2.0.[15]
For a white American woman at age 50, the risk of suffering an osteoporotic fracture in her remaining lifetime has been estimated at 40%,[16] with two thirds of the fractures occurring after age 75.[17] The estimated remaining lifetime risks after age 50 for hip, vertebral, and forearm fracture are 17.5%, 15.6%, and 16.0%, respectively.[16]
In the United States, the rates of osteoporosis and fracture vary with ethnicity. In one large study of postmenopausal women from five ethnic groups (white Americans, African Americans, Asian Americans, Hispanic Americans, and Native Americans),[18] African Americans had the highest BMD, whereas Asian Americans had the lowest; only the BMD differences for African Americans were not explained by differences in weight. After adjusting for weight, BMD, and other covariates, white Americans and Hispanic Americans had the highest risk for osteoporotic fracture, followed by Native Americans, African Americans, and Asian Americans. The age-adjusted lifetime risks of hip fracture in US women are 17% for white Americans, 14% for Hispanic Americans, and 6% for African Americans.[11] These differences, however, may be related more to body size than to race.[12,19]
Canadian data on hip fractures is reliably collected from hospital discharges. An analysis showed declining age-adjusted hip fracture incidence (decreases of 31.8% in women and 25% in men) over the 21 years of the study.[20]
Morbidity and Mortality
Hip fractures, which occur on average at age 82, elicit a particularly devastating toll, resulting in higher cost, disability, and mortality than all other osteoporotic fracture types combined. Hip fractures cause up to a 25% increase in mortality within 1 year of the incident. Approximately 25% of women require long-term care after a hip fracture, and 50% will have some long-term loss of mobility.
Fractures at other sites can also result in serious morbidity. Vertebral fractures occur, on average, in a woman's mid-70s. Multiple or severe vertebral fractures may cause substantial pain as well as loss of height and exaggerated thoracic kyphosis (abnormal curvature of the thoracic spine). Spinal pain and deformity can greatly restrict normal movement, including bending and reaching. Importantly, existing vertebral fractures greatly increase (at least five- to sevenfold) the risk of subsequent vertebral fracture.[21,22] Thoracic fractures may restrict lung function and cause digestive problems.[23] In the Fracture Intervention Trial,[24] after an average of 3.8 years of follow-up, the relative risk (RR) for mortality was 6.7 (95% CI, 3.08–14.52) for hip fracture and 8.64 (95% CI, 4.45–16.74) for vertebral fracture.
Osteoporotic fractures take a psychological toll as well.[25] Hip and vertebral fractures and the resultant pain, loss of mobility, changed body image, and loss of independence can have a strong impact on self-esteem and mood.
Pathophysiology
Bone remodeling is a coupled process of bone resorption followed by bone formation. At the cellular level, osteoclasts promote bone resorption by stimulating the production of acid and enzymes that dissolve bone mineral and proteins. Osteoblasts promote bone formation by creating a protein matrix consisting primarily of collagen that is soon calcified, resulting in mineralized bone.
In normal bone remodeling, bone resorption is balanced by bone formation. Bone loss occurs when there is an imbalance between bone resorption and bone formation, resulting in a decrease in bone mass and an increase in the risk of fracture.
Menopause is associated with a few years of rapid bone loss attributed to lower circulating levels of 17β-estradiol, related primarily to the loss of estrogen-mediated inhibition of bone resorption without a fully compensatory increase in bone formation.[26] However, there is only a weak association between serum estradiol levels and rates of bone turnover in postmenopausal women.
Clinical Risk Factors
In determining risk factors, it is important to distinguish between risk factors for osteoporosis as defined by BMD (both primary and secondary causes) and risk factors for osteoporotic fracture. For BMD-defined osteoporosis, major risk factors in postmenopausal women are advanced age, genetics, lifestyle factors (eg, low calcium and vitamin D intake, smoking), thinness, and menopause status. The most common risk factors for osteoporotic fracture are listed in Table 1.
Table 1. Risk factors for osteoporotic fracture used in FRAX®
|
a Body mass index is automatically computed from height and weight.
Adapted from World Health Organization Collaborating Centre for Metabolic Bone Diseases.[28]
In the absence of other risk predictors such as BMD, clinical risk factors can be used to assess fracture risk or help make the decision as to which women should be screened with dual-energy x-ray absorptiometry (DXA). Such risk factors increase the risk of fracture 1.5- to 3-fold over that seen in unaffected individuals. Women with multiple risk factors are at greater risk of fracture if they have a lower BMD. The use of BMD T-scores to assess fracture risk can be markedly improved by combining BMD with information about other risk factors, particularly the woman's age and fracture history.
Although there is good evidence that many clinical risk factors can increase fracture risk, it is less clear which of these have an effect separate from their effect on bone density. Therefore, clinical risk factors could help us improve fracture risk reduction, but which factors to choose and how to integrate them must still be established.
Recently, WHO conducted a meta-analysis of the relationship of clinical risk factors and fracture using global epidemiology data from 12 cohorts with approximately 250,000 person-years, 60,000 patients, and over 5,000 fractures, which was confirmed in 11 additional cohorts.[27] Candidate risk factors were chosen based on availability of global data, independence of the risk factor from BMD, ease of use in clinical practice, responsiveness to pharmaceutic intervention, and intuitive use in clinical care. A total of 10 risk factors were identified that met these criteria. The risk factors were then used to create a platform called FRAX® to calculate the 10-year risk of major osteoporotic fracture (hip, shoulder, wrist, and clinical spine). Note that the Canadian FRAX model is not yet available, but clinicians can use a model from a country with similar ethnicity and demographics. (See the section on "Evaluation" for more about FRAX.)
Bone Mineral Density and Fracture Risk
BMD is an important determinant of fracture risk, especially in women age 65 and older.[29,30]
In general, lower BMDs are associated with a higher risk of fracture. A decrease of 1 SD in BMD represents a 10% to 12% decrease in BMD and an increase in fracture risk by a factor of 1.5 to 2.6, depending on fracture type and measurement.[31,32] BMD and fracture risk are most closely related when BMD is used to predict the fracture risk at that same site. Risks for spine fracture and hip fracture increase 2.3-fold and 2.6-fold, respectively, for each decrease of 1 SD in age-adjusted BMD at spine and hip, respectively.[31] The risk of any fracture increases 1.6-fold with each SD in age-adjusted BMD at the hip. The gradient of risk (RR per SD) is higher at a younger than an older age and decreases markedly with age. For example, the gradient of risk for hip fracture is 3.68 per SD change in hip BMD at age 50, decreasing progressively with age until at age 85 it is 1.93 per SD.[33] Although epidemiology studies have examined BMD in both the femoral neck and total hip, the two regions may be able to be used interchangeably, but no clear-cut priority is indicated.[33]
Treatment-induced changes in BMD do not always correlate well with reductions in vertebral fracture risk.[34–37] In addition, fracture risk reductions in response to antiresorptive therapy occur much more rapidly than discernible BMD changes. For example, significant fracture risk reduction has been reported after 6 months of risedronate therapy,[38] although minimal BMD increases were observed at that time.[39]
Age
As women age, their risk for fracture increases. In general, the risk of osteoporotic fracture doubles every 7 or 8 years after age 50. The median age for hip fracture is 82 years. The median age for vertebral fracture is thought to occur in a woman's 70s.[12]
Age is a particularly strong risk factor for fracture, particularly hip fracture. Based on BMD alone, it would be expected that the hip fracture risk would increase fourfold between ages 55 and 85. However, age increases hip fracture risk up to 40-fold over that three-decade time span. Thus, the impact of increasing age is much greater, or at least 10-fold greater, than the impact of a decreasing BMD.[34] For example, using FRAX 3.0, a patient at age 50 with a femoral neck DXA T-score of −1.5 has a 10-year hip fracture probability of approximately 2.5%, but at age 80, the probability is approximately 7% with the same T-score at the same site.[34] For any osteoporotic fracture, the 10-year probability with a T-score of −2.5 SD at the femoral neck varies from 7% at age 50 to 20% at age 80.[34]
Fracture History
It is well established from many cohort, case-control, and cross-sectional studies that a prior osteoporotic fracture increases the risk of future fractures. A prior forearm fracture is associated with a twofold increase in subsequent risk of fracture. In two analyses of studies, a peri- or postmenopausal woman who has had a fracture has approximately a twofold increased risk of sustaining another fracture; adjustment for BMD did not significantly affect the risk.[22,40] When the placebo group in randomized controlled trials (RCTs)[41,42] is examined, the risk of future vertebral deformities over the 3 years of the trials is fivefold higher in patients with prior vertebral deformity than in those without. A study of older women (mean age, 74 y) with recent vertebral fracture found that approximately 20% of these women experienced another vertebral fracture within 1 year of an incident vertebral fracture.[21] However, the risk of recurrent fracture was significantly affected by the number of existing fractures—women with two or more vertebral fractures had a significantly increased risk (RR, 11.6) of another vertebral fracture within 1 year.
This increased fracture risk may be in part attributable to lower BMD in patients who have had fractures. However, when the increased risk is adjusted for BMD, the RR is adjusted only slightly lower. The risk ratio is only marginally lowered (~10%) when BMD is taken into account, arguing that the presence of a fracture is a powerful marker of impaired bone quality above and beyond BMD.[43]
Genetics
The greatest influence on a woman's peak bone mass (ie, the maximal BMD gained during the skeletal development and maturation phase) is heredity. Studies have suggested that up to 80% of the variability in peak BMD might be attributable to genetic factors.[44,45] Daughters of women who have osteoporotic fractures have lower BMD than would be expected for their age.[46,47] First-degree relatives (ie, mother, sister) of women with osteoporosis also tend to have lower BMD than those with no family history of osteoporosis.[48]
A history of fracture in a first-degree relative also significantly increases the fracture risk. In a meta-analysis,[49] a family history of fracture was found to be associated with significant increases in any osteoporotic fracture. Hip fracture risks were nearly 50% higher—127% higher if a hip fracture had occurred in a parent. Risk ratios were slightly higher for hip fracture (RR, 1.63) than for any fracture (RR, 1.18) or for any osteoporotic fracture (RR, 1.22). A parental history of hip (rather than any) fracture gives a risk ratio for any fracture of 1.42, similar to that of any osteoporotic fracture (RR, 1.54); the highest risk was of hip fracture (RR, 2.27). Inasmuch as patient recall of parental hip fracture is higher than of any fracture, parental hip fracture was chosen as a clinical risk factor in FRAX.[28]
Lifestyle Factors
Several lifestyle factors are associated with the risk of low BMD and fracture. These include poor nutrition, insufficient physical activity, cigarette smoking, and heavy alcohol consumption. (For a complete description of osteoporosis lifestyle factors, see section on "Management: Lifestyle approaches.")
Body Mass Index and Thinness
Being thin—often cited as body weight under 127 lb (57.7 kg), the lower quartile of weight for US women over age 65, or a body mass index (BMI) less than 21 kg/m2—is a risk factor for low BMD.[50] Thinness has also been associated with increased fracture risk, especially in older women.[51] Low weight or low BMI is a well-documented risk factor for future fracture, whereas high BMI may be protective. Although the risk of fracture increases with decreasing BMI, the risk ratio with BMI is nonlinear.[33] The risk ratio is markedly higher at the lower values of BMI, particularly at a BMI of 20 kg/m2 or less. By contrast, between a BMI of 25 kg/m2 and 35 kg/m2, the differences in risk ratio are smaller. There appears to be an inflection point at which increased BMI over 22 kg/m2 is associated with modest decreases in fracture risk, whereas the risk is considerably increased below that threshold.[33] This gradient of risk with BMI is greatly reduced when adjusting for BMD, suggesting that BMD is an important intermediary or confounder. However, when BMD is not available, low BMI may be used to identify populations with low BMD and high risk of fracture. In FRAX, low BMI is used when BMD is not available.[33]
Menopause Status
The increased rate of bone resorption immediately after menopause clearly indicates a hormonal influence on bone density in women. The most likely explanation for this increased resorption is the drop in ovarian estrogen production that accompanies menopause.
Bone loss begins to accelerate approximately 2 to 3 years before the last menses, and this acceleration ends 3 to 4 years after menopause. For an interval of a few years around menopause, women lose 2% of bone annually. Afterward, bone loss slows to about 1% to 1.5% per year.[52,53] A prospective, longitudinal study of white women reported BMD losses during this 5- to 7-year interval of 10.5% for the spine, 5.3% for the femoral neck, and 7.7% for the total body.[52] Although some of the decline can be attributed to age-related factors, lower estrogen levels were implicated as the cause for approximately two thirds of the bone loss. Lower estrogen levels have also been significantly associated with increased fracture risk in older women (mean age, 75 y).[54]
Women experiencing menopause at or before age 40—either spontaneously or induced (eg, through bilateral oophorectomy, chemotherapy, or pelvic radiation therapy)—are at greater risk of low BMD than other women of the same age who have not reached menopause.[55] However, by age 70, when fractures are more likely to occur, these women have the same risk for low BMD or fracture as women who reached menopause at the average age.[56,57]
Secondary Causes of Bone Loss
Various medications, disease states, and genetic disorders are associated with bone loss (Table 2). There is some early evidence that certain disease states may provide a risk of fracture over and above that provided by BMD. These disorders include hyperthyroidism, type 1 diabetes, ankylosing spondylitis, and rheumatoid arthritis (RA), among others.[33] However, due to the absence of data for secondary osteoporosis, FRAX currently uses RA as a significant surrogate risk factor for any fracture (RR, 1.45), osteoporotic fracture (RR, 1.56), and hip fracture (RR, 1.95). This risk persists after adjustment for glucocorticoid use, BMD, and prior fracture.[33] Vertebral fracture risk is approximately twofold higher in RA patients than in controls and independent of BMD and prior glucocorticoid use.[58]
Table 2. Secondary causes of bone loss
| Medications |
| Aromatase inhibitors |
| Cytotoxic agents |
| Excessive thyroxine doses |
| Gonadotropin-releasing hormone agonists or analogues |
| Heparin |
| Immunosuppressives (eg, cyclosporine) |
| Intramuscular medroxyprogesterone |
| Long-term use of certain anticonvulsants (eg, phenytoin) |
| Oral or intramuscular use of glucocorticoids for >3 mo |
| Genetic disorders |
| Hemochromatosis |
| Hypophosphatasia |
| Osteogenesis imperfecta |
| Thalassemia |
| Disorders of calcium balance |
| Hypercalciuria |
| Vitamin D deficiency |
| Endocrinopathies |
| Cortisol excess |
| Cushing_s syndrome |
| Gonadal insufficiency (primary and secondary) |
| Hyperthyroidism |
| Primary hyperparathyroidism |
| Type 1 diabetes mellitus |
| Gastrointestinal diseases |
| Billroth I gastroenterostomy |
| Chronic liver disease (eg, primary biliary cirrhosis) |
| Malabsorption syndromes (eg, celiac disease, Crohn_s disease) |
| Total gastrectomy |
| Other disorders and conditions |
| Ankylosing spondylitis |
| Chronic renal disease |
| Lymphoma and leukemia |
| Multiple myeloma |
| Nutritional disorders (eg, anorexia nervosa) |
| Rheumatoid arthritis |
| Systemic mastocytosis |
There is strong evidence that certain medications such as oral glucocorticoids result in BMD loss and increased risk of fracture. Other studies[59,60] suggest that no BMD loss occurs with the approved doses of inhaled steroids. Epidemiologic data suggest that the risk of hip, forearm, and shoulder fractures is increased approximately twofold in patients taking glucocorticoids. The risk of vertebral fracture may be higher. In the largest study examining fracture risks,[61] approximately 250,000 glucocorticoid users were matched with age and sex controls. A dose-dependent effect was noted with a dose of prednisolone or equivalent greater than 7.5 mg/day (daily, RR of vertebral fracture, 5.2), whereas with 5.0 to 7.5 mg/day, the risk was lower (RR, 2.6). Ever-use of glucocorticoids has been associated with significant increased risk of any fracture at all ages compared with the risk faced by people with no glucocorticoid exposure.[33] This discrepancy is not explained by BMD. For example, for individuals at age 50, the RR for any fracture with glucocorticoids was 1.9; similarly, RR for any fracture was 1.98 when adjusted for BMD. The data strongly suggest that risk of all fractures is substantially greater in glucocorticoid-induced osteoporosis than in postmenopausal osteoporosis at the same level of BMD.
Two drugs currently prescribed for premenopausal women—gonadotropin-releasing hormone (GnRH) agonists and intramuscular depot medroxyprogesterone acetate (MPA)—have been associated with bone loss. GnRH agonists contributes to bone loss by creating iatrogenic hypogonadism.[62] Bone loss with short-term use of GnRH agonist therapy is reversible. Bone loss with long-term use can be ameliorated by "adding back" low-dose estrogen therapy (ET). Use of depot MPA (150 mg/3 months) as a contraceptive has been associated with bone loss.[63,64] This bone loss, which has never been linked to the occurrence of osteoporotic fracture, has been shown in some studies to be reversible; however, other studies have indicated that BMD only partially recovers.
Aromatase inhibitors used for breast cancer treatment have also been associated with bone loss.[65] Breast cancer patients are at increased risk of clinical fracture compared with the general postmenopausal population and aromatase inhibitors have a slight additive effect on fracture risk (eg, anastrozole [RR, 1.36]) over 5 years.[66]
Medical conditions also associated with bone loss include excess urinary calcium excretion, which may be caused by a renal calcium leak or hyperthyroidism. Vitamin D deficiency, an especially common condition in older women, is a correctable cause of secondary hyperparathyroidism and accelerated bone loss. Other conditions that can have a detrimental effect on bone include multiple myeloma, endocrine disorders such as hyperparathyroidism and Cushing's syndrome, and disorders of collagen structures. Renal failure can cause either increased bone resorption (secondary/tertiary hyperparathyroidism) or decreased bone formation, leading to renal osteodystrophy.
Other Potentially Important Risk Factors
Recent reviews suggest that the use of biochemical indices of bone turnover may be a possible predictor of fracture risk in postmenopausal osteoporosis.[67] A recent review of prospective and cross-sectional studies concludes that increased bone resorption markers were associated with increased fracture risk,[68] but global data are not available to enable the use of bone markers in FRAX. (For more about bone turnover markers, see the section on "Evaluation.")
According to the Canadian Multicentre Osteoporosis Study, bone loss as documented by changes in BMD over time is associated with increased risk of fracture.[69] It is not, however, included in the FRAX calculator due to lack of global data.
FRAX uses a history of prior clinical fracture as a clinical risk factor. A prior morphometric vertebral fracture, documented in three cohorts, is associated with increased risk for subsequent osteoporotic fracture (RR, 2.27) and for hip fracture (RR, 2.68).[33] For this reason, the term "prior fracture" should take into account not only clinical vertebral but morphometric vertebral fractures as well.
Limitations of using Risk Factors in Predicting Fracture
It is important to recognize that the strength of a risk factor varies according to fracture outcome. In general, risk factors are more strongly associated with hip fracture risk than the risk of any osteoporotic fracture. Thus, current models usually calculate hip fracture risk separately from risk of other osteoporotic fractures.
Existing studies often do not take into account dose response, but give risk ratios for an average dose or exposure. There is good evidence, however, that the risk associated with excess alcohol or overuse of glucocorticoids is dose responsive.[70] In addition, the risk of fracture increases progressively with number of prior fractures.[33]
Evaluation
All postmenopausal women should be assessed for risk factors associated with osteoporosis and fracture. This assessment requires a history, physical examination, and any necessary diagnostic tests. The goals of this evaluation are to evaluate fracture risk, to rule out secondary causes of osteoporosis, to identify modifiable risk factors, and to determine appropriate candidates for pharmacologic therapy.
History and Physical Examination
The medical history and physical examination should solicit clinical risk factors for osteoporosis and fracture and also evaluate for secondary causes of osteoporosis and fragility fracture. This includes the WHO's FRAX risk factors (personal history of fracture after age 40, history of hip fracture in a parent, cigarette smoking, excess alcohol consumption, glucocorticoid use, RA, or other secondary causes of osteoporosis. See Table 1). Risk factors must be accurately collected, often with the aid of a simple questionnaire. Risk factors may help identify contributing causes of osteoporosis and are essential in the determination of FRAX. This tool, used with guidelines for treatment thresholds, is very helpful in identifying candidates for pharmacotherapy. Osteoporosis can be diagnosed by bone density testing in postmenopausal women over age 50. A fragility fracture can also indicate a clinical diagnosis of osteoporosis.
Table 1. Risk factors for osteoporotic fracture used in FRAX®
|
a Body mass index is automatically computed from height and weight.
Adapted from World Health Organization Collaborating Centre for Metabolic Bone Diseases.[28]
Loss of height and kyphosis may be signs of vertebral fracture. After achieving maximal height, women can lose up to 1.0 to 1.5 inches (2.0–3.8 cm) of height as part of the normal aging process, primarily as a result of degenerative arthritis and shrinkage of intervertebral disks. Height loss greater than 1.5 inches (3.8 cm) increases the likelihood that a vertebral fracture is present.[71] Height should be measured annually with an accurate method, such as a wall-mounted ruler or a stadiometer. Loss of 1.5 inches (3.8 cm) or more calls for evaluation by a lateral thoracolumbar radiograph or vertebral fracture assessment (VFA) by DXA to identify vertebral fractures.
Weight should also be recorded to identify those women with low BMI and to be aware of weight changes, which may interfere with the interpretation of changes in BMD over time.
The evaluation should include eliciting symptoms of acute or chronic back pain, which may indicate the presence of vertebral fractures. Signs of percussion tenderness may indicate acute fracture or bony infiltrative disease. The midback vertebrae T11-T12 and L1 are the most common fracture sites, followed by T6 through T9.[72–74] Vertebral compression fractures may result in kyphosis, the most obvious sign of osteoporosis.
Because back pain, height loss, and kyphosis can occur without osteoporosis, and because two thirds of vertebral fractures are asymptomatic,[75,76] vertebral fracture must be confirmed by lateral spine radiographs or VFA visualization of fracture at the time of BMD testing.[77,78] Vertebral height loss of more than 20%—more than 2 mm (measured) or 4 mm (historical)—of the anterior, mid, or posterior dimension of a vertebra on imaging is indicative of vertebral fracture.[79,80] Grading of vertebral fractures and percentage of height reduction (grade 1, mild, 20%–25%; grade 2, moderate, 25%–40%; grade 3, severe, over 40%) by a Genant semiquantitative methodology or equivalent is most important in the evaluation of the patient with severe osteoporosis. Both the number and the severity of existing vertebral fractures predict the risk of future fracture.
After menopause, a woman's risk for falls should be assessed. Clinical factors related to an increased risk of falls include the following:
- A history of falls, fainting, or loss of consciousness
- Muscle weakness
- Dizziness, coordination, or balance problems
- Difficulty standing or walking
- Arthritis of the lower extremities
- Neuropathy of the lower extremities
- Impaired vision
The risk of falls is also increased by use of medications that affect balance and coordination (eg, sedatives, narcotic analgesics, anticholinergics, antihypertensives) or by use of multiple medications.[81]
The greater the number of risk factors, the greater the risk of falling. In one study, having four or more of these risk factors increased the risk of falls by nearly 80%.[82] Several studies have indicated that exercise and gait/balance training may decrease the risk of falls.[83,84]
Safety hazards in the home and work environment, such as obstacles and poor lighting, also contribute to the risk of falls. These hazards can be assessed by questioning the woman or through a home or workplace visit (or both) by an occupational therapist or other healthcare professional knowledgeable about fall prevention.
BMD Measurement
BMD testing of hip (femoral neck, total hip), spine (at least two vertebral bodies), or radius (one-third radius site) is required for a densitometric diagnosis of osteoporosis. Measurements of bone strength other than bone density at these sites may predict fracture risk but cannot be used to diagnose osteoporosis. A clinical diagnosis of osteoporosis can be made if fragility fractures are present, regardless of the BMD.
Indications for BMD Testing The decision to test BMD in a postmenopausal woman should be based on the woman's risk profile. Testing is not indicated unless the results will influence a management decision. Although perimenopausal women can be classified by WHO criteria and may be candidates for FRAX risk assessment, care must be taken to appropriately interpret DXA tests and to make correct recommendations for risk factor reduction and sometimes pharmacotherapy. Other factors, such as availability of BMD testing equipment and reimbursement by insurance, also affect the decision to measure BMD.
NAMS recommends that BMD be measured in the following populations:
- All women age 65 and over, regardless of clinical risk factors
- Postmenopausal women with medical causes of bone loss (eg, steroid use, hyperparathyroidism), regardless of age
- Postmenopausal women age 50 and over with additional risk factors (see below)
- Postmenopausal women with a fragility fracture (eg, fracture from a fall from standing height)
Testing should be considered for postmenopausal women age 50 and over when one or more of the following risk factors for fracture have been identified:
- Fracture (other than skull, facial bone, ankle, finger, and toe) after menopause
- Thinness (body weight < 127 lb [57.7 kg] or BMI < 21 kg/m2)
- History of hip fracture in a parent
- Current smoker
- Rheumatoid arthritis
- Alcohol intake of more than two units per day (one unit is 12 oz of beer, 4 oz of wine, or 1 oz of liquor)
Bone-testing Options Fracture risk can be estimated by a variety of technologies at numerous skeletal sites. BMD measured by DXA is the only diagnostic technology by which measurements are made at hip, spine, and radius. These are also important sites of osteoporotic fracture.[85]
When BMD testing is indicated, NAMS recommends measuring the total hip, femoral neck, and posterior-anterior lumbar spine, using the lowest of the three BMD scores for diagnosis. In some patients, degenerative or other artifacts at the spine site make measurements unreliable. In such cases, the one-third radius should be measured and used as a second site valid for diagnosis. The spine may be a useful site for BMD measurement in early postmenopausal women because decreases in BMD can be faster at the spine than at the hip.
Although bone tests at peripheral sites (eg, tibia, finger, calcaneus) can identify women at risk of fracture, they are not useful for the diagnosis of osteoporosis and have limited or no value in the follow-up of patients.[86] Peripheral site measurements may be useful to raise awareness about bone health and have been utilized as a prescreen for DXA testing where DXA availability is limited.[87]
Follow-up BMD Testing In most cases, repeat DXA testing in untreated postmenopausal women is not useful until 2 to 5 years have passed, given the rate of bone loss of 1% to 1.5% per year. Postmenopausal women, after substantial BMD losses in early postmenopause, generally lose about 0.5 T-score units in BMD every 5 years.[51,88]
For women receiving osteoporosis therapy, BMD monitoring may not provide clinically useful information until after 1 to 2 years of treatment. Stable BMD (within the precision error of the instrument) indicates successful therapy; fracture risk reductions for patients on antiresorptive therapy are similar with stable bone density or with increases in BMD. Marked declines in BMD predict greater fracture risk and should trigger a reevaluation for secondary causes of osteoporosis or treatment nonadherence.
Each DXA testing center should perform precision testing to determine the least significant change that can be detected in their patient population. Statistically insignificant decreases in BMD should be reported as stable bone density within the precision error of the instrument. Statistically significant changes in BMD (equal to or greater than the least significant change) should be reported as such.
Bone Turnover Markers
Biochemical markers of bone turnover can be measured in serum or urine. They can indicate either osteoclastic bone resorption (breakdown products of type I collagen in bone: N-telopeptides, C-telopeptides, deoxypyridinoline) or osteoblast functioning (bone matrix synthesis: bone-specific alkaline phosphatase, procollagen type I N-terminal propeptide, osteocalcin). Bone turnover markers cannot diagnose osteoporosis and have varying ability to predict fracture risk when studied in groups of patients in clinical trials.[89,90] They also have varying value in predicting individual patient response to therapy. Nevertheless, these tests may show an individual patient's response to therapy earlier than BMD changes, sometimes within 2 to 3 months as opposed to the 1 to 3 years required with BMD.[91,92] Most bone turnover markers vary greatly from day to day, are affected by food intake and time of day, and lack assay standardization, limiting their clinical utility. In some cases, persistently elevated bone turnover markers in the face of antiresorptive therapy may alert the clinician to nonadherence to therapy, poor absorption of medication, or other secondary causes of osteoporosis.
The value of bone turnover markers in encouraging adherence to therapy has been debated. Several trials have found no difference in adherence when marker values are communicated to women.[93,94]
Tests for Secondary Causes
Low BMD in postmenopausal women is most often the result of low peak bone mass, postmenopausal declines in bone density (related to estrogen deficiency), or both. There are, however, important secondary causes of bone loss, which should be identified clinically and through appropriate laboratory testing. Laboratory tests that may be useful in some circumstances are listed in Table 3. Routine tests for patients with low bone mass include a complete blood cell count, serum calcium, phosphate, creatinine, thyroid-stimulating hormone, alkaline phosphatase, and albumin. Tests for serum 25-hydroxyvitamin D [25(OH)D] and 24-hour urinary calcium excretion may be useful to detect patients with poor calcium and vitamin D nutrition as well as those with hypercalciuria. Special tests that may be appropriate in some clinical circumstances include 24-hour urine free cortisol, serum protein electrophoresis, tissue transglutaminase antibody, and intact parathyroid hormone (PTH).
Table 3. Routine laboratory tests for osteoporosis evaluation
| Test | Diagnostic result | Possible secondary cause |
|---|---|---|
| Complete blood cell count | Anemia | Multiple myeloma |
| Serum calcium | Elevated | Hyperparathyroidism |
 |
Low | Vitamin D deficiency, GI malabsorption |
| Serum phosphate | Elevated | Renal failure |
 |
Low | Hyperparathyroidism |
| Serum 25-hydroxyvitamin D | Low | Undersupplementation, GI malabsorption, celiac disease |
| Serum albumin | Used to interpret serum calcium, nutritional deficiencies |  |
| Serum alkaline phosphatase | Elevated | Vitamin D deficiency, GI malabsorption, hyperparathyroidism, Paget's disease, liver/biliary disease |
| Urinary calcium excretion | Elevated | Renal calcium leak, multiple myeloma, metastatic cancer involving bone, hyperparathyroidism, hyperthyroidism |
 |
Low | GI malabsorption, inadequate intake of calcium and vitamin D |
| TSH | Low | Hyperthyroidism (causes excess bone turnover) |
 |
High | Hypothyroidism |
| Serum protein electrophoresis | Monoclonal band | Multiple myeloma |
| Tissue transglutaminase antibody (gluten enteropathy) | Elevated | Predictive of celiac disease |
| Creatinine | Elevated | Renal osteodystrophy, possible contraindication to bisphosphonates |
GI, gastrointestinal; TSH, thyroid-stimulating hormone.
Management: Lifestyle Approaches
Lifestyle approaches alone may not be sufficient to prevent bone loss or reduce fracture risk, but they form the necessary foundation for pharmacologic approaches to the prevention or management of osteoporosis. In some cases, recommended lifestyle approaches may be sufficient. All postmenopausal women, regardless of their bone density or clinical risk factors for osteoporosis, should be encouraged to eat a balanced diet, obtain adequate calcium and vitamin D, participate in appropriate exercise, avoid cigarette smoke and excessive alcohol consumption, and institute fall prevention measures. These recommendations offer health benefits beyond their effects on the prevention or management of osteoporosis. The recommendations are, in fact, so obvious that their importance may not be appreciated. The success of these approaches is heavily dependent on patient education and motivation to institute them.
Nutrition
A balanced diet is important for bone development and maintenance, as well as for general health. Some populations, such as women over age 65, edentulous women, women with reduced appetites from any cause, or women who diet frequently or have eating disorders, may not consume adequate vitamins and minerals to maintain optimal bone mass. Older women who lose weight, purposely or not, run the risk of accelerated bone loss and a higher risk of hip fracture.[95] Based on the US Department of Agriculture's Healthy Eating Index (HEI) score, women age 60 and older in the United States do not consume the recommended servings of dairy products, fruits, vegetables, or grains. The overall HEI score for such women was 67.4 out of a possible 100, indicating dietary habits in need of improvement.[96] In the specific context of the prevention and management of osteoporosis, a discussion of nutrition appropriately focuses on calcium and vitamin D, vitamin K, magnesium, protein, and isoflavones.
Calcium and Vitamin D Nutritional issues of calcium and vitamin D are perhaps the most important. An adequate intake of both calcium and vitamin D is important for bone health and is recognized as an important component of any osteoporosis prescription-drug regimen. Indeed, as part of the US Food and Drug Administration's approved labeling of all bisphosphonates used for the prevention and treatment of postmenopausal osteoporosis, correction of disorders of mineral metabolism such as calcium and/or vitamin D deficiency is mandatory before initiating therapy. Calcium and vitamin D supplements, however, should not be substituted for a prescription intervention when deemed necessary.
Calcium. Calcium, a mineral, is generally deficient in North American diets because of the relatively limited, concentrated sources of dietary calcium. Compounding this issue is that, compared with other minerals, the daily requirement for calcium is large. Calcium can generally be viewed as a weak antiresorptive agent as well as an essential nutrient. Evidence has established the role of adequate calcium intake in bone health, primarily in the development of peak bone mass and in preventing bone loss. The evidence for calcium's ability to reduce fracture risk is not as strong. However, in a 5-year, double-blind, placebo-controlled trial of postmenopausal women with a mean age of 75 years, the 830 women who were compliant with their calcium supplements had a significant reduction in the hazard ratio for fracture of 0.66.[97] So many other trials have involved a combination of calcium and vitamin D that it is difficult to separate the effects of the two. For example, in the Women's Health Initiative (WHI) trial,[98] hip fractures were significantly reduced in older women who were adherent to the calcium and vitamin D regimen.
The primary factor influencing the amount of calcium available for absorption is the amount of calcium ingested. Unfortunately, data suggest that daily calcium intake tends to decline with advancing age.[99] Additionally, intestinal transport studies suggest that for any given luminal concentration of calcium, intestinal absorption of calcium is less in older women than young.[100] Vitamin D deficiency, now recognized as exceedingly widespread, will contribute as well to declining calcium absorption.[101,102] Renal insufficiency may result in 1,25-dihydroxyvitamin D deficiency quite independently of inadequate sun exposure or vitamin D intake. Estrogen deficiency also appears to result in an increase in urinary calcium excretion.[103]This combination of circumstances necessitates an increase in the daily calcium intake in women over age 50 and in the setting of estrogen deficiency.
Most experts support the published recommendations for total daily calcium consumption from the National Osteoporosis Foundation (NOF),[104] the National Institutes of Health,[105] the National Academy of Sciences (NAS),[106] or Osteoporosis Canada.[107] Recommendations for perimenopausal and postmenopausal women are presented in Table 4.
Table 4. Recommended daily elemental calcium intake in peri- and postmenopausal women
| National Osteoporosis Foundation | |
| Women age 50 and over | 1,200 mg |
| National Institutes of Health | |
| Premenopausal women ages 25–50 | 1,000 mg |
| Postmenopausal women younger than age 65 and using estrogen therapy | 1,000 mg |
| Postmenopausal women not using estrogen therapy | 1,500 mg |
| All women age 65 and older | 1,500 mg |
| National Academy of Sciences | |
| Age 31–50 | 1,000 mg |
| Age 51 and older | 1,200 mg |
| Osteoporosis Canada | |
| Women over age 50 | 1,500 mg |
Adapted from the National Osteoporosis Foundation 2008,[104] National Institutes of Health 1994,[105] National Academy of Sciences 1997,[106] and Osteoporosis Canada.[107]
Based on data from the National Health and Nutrition Survey 1999–2000, US women ages 40 to 59 and age 60 and older have mean calcium intakes from dietary sources of 744 mg and 660 mg, respectively.[99] Mean daily dietary calcium intake in Canadian women ages 50 to 70 is reported to be 740 mg.[108] Thus, the average postmenopausal woman in the United States or Canada can reasonably be assumed to consume a diet that is approximately 500 mg less than the recommended 1,200 mg/day. Specific populations of postmenopausal women at increased risk for inadequate calcium intake include women who are older, are lactose intolerant, follow a vegetarian diet, or have poor eating habits. No single laboratory test can accurately detect calcium deficiency. However, a 24-hour urine calcium level of less than 50 mg suggests either insufficient intake or poor absorption.
Dietary sources of calcium, although limited, are recommended as the primary source of calcium because of the other essential nutrients found in high-calcium foods. Dairy products are the major contributors of dietary calcium, providing approximately 80% of total calcium intake of postmenopausal women age 60 and older.[109] Dairy products also tend to be the best dietary sources of calcium because of their high elemental calcium content, high absorption rate, and low cost relative to total nutritional value. To achieve maximal calcium absorption from food sources, food selection decisions should reflect the food's calcium bioavailability and the presence in the meal of other foods that may inhibit calcium absorption (eg, oxalic acid-containing foods such as spinach, and phytate-rich grains such as wheat bran).[110]
Calcium supplements and calcium-fortified foods are additional sources of calcium for women unable to consume sufficient dietary calcium; most women will need an additional 600 to 900 mg/day over their usual daily intake to reach recommended levels. Calcium supplements are available in a variety of different calcium salts, such as calcium carbonate or calcium citrate. The specific salt tends to determine the size of the tablet and the concentration of elemental calcium in the tablet. For example, a 1,250-mg calcium carbonate tablet will contain 500 mg of elemental calcium.
The calcium salt may also affect the circumstances surrounding administration. Calcium citrate supplements are well absorbed when taken with meals or on an empty stomach; calcium carbonate is better absorbed when taken with food. In all cases, it is best to take calcium in divided doses for better absorption.
Total calcium intakes of up to 1,500 mg/day do not appear to increase the risk of developing renal calculi and may actually reduce it.[111] There appears to be no benefit to consumption of amounts in excess of 1,500 mg/day. Calcium supplements are contraindicated in a woman with a calcium-containing renal calculus until her urinary biochemical profile has been assessed. The NAS has established the upper limit of tolerable intake for calcium for adults as 2,500 mg/day. Larger amounts of calcium should be avoided.
Total daily intake recommendations for calcium refer to elemental calcium only. The amount of elemental calcium that is needed in a supplement is the difference between the total recommended intake and the dietary consumption of elemental calcium.
Calcium intervention trials have not reported any serious adverse events. In one clinical trial in which 600 mg of elemental calcium was given twice a day as calcium carbonate, constipation was the only adverse event occurring more commonly in the treated group than in the group receiving placebo.[97] Some women have difficulty swallowing a calcium supplement if the tablet is large or they have other gastrointestinal (GI) adverse effects such as bloating or increased flatus. Tolerability can be addressed by using achewable or liquid calcium supplement, changing the type of calcium salt, or by reducing the dose. GI adverse effects may be related to the specific calcium salt, taking more calcium than required, or not dividing doses.
Vitamin D. Vitamin D is actually a steroid prohormone rather than a vitamin, as it can be produced in the human body through the interaction of sunlight with the skin. Nevertheless, this nutrient is commonly characterized as a vitamin. It is essential for the physiologic regulation and stimulation of intestinal absorption of calcium.[112] The 1997 NAS-recommended dietary allowance (RDA) for vitamin D is 400 IU/day for women ages 51 to 70 and 600 IU/day for women older than age 70.[106]Current expert opinion, however, is that this intake level is inadequate to maintain vitamin D deficiency for optimum bone health.[113,114] NOF recommends that postmenopausal women obtain 800 to 1,000 IU of vitamin D/day.[104] In Canada, the recommended intake for women under age 50 is 400 IU/day and 800 IU/day for women over age 50[107] (an upward revision is being considered).
Although vitamin D is produced from the interaction of ultraviolet rays from sunlight with 7-dehydrocholesterol in the skin, use of a sunscreen with a sun protection factor of 8 or higher will block the production of vitamin D by 97.5%.[115] Unprotected exposure of the skin to sunlight is not recommended as a means of addressing vitamin D deficiency.[116] Darker skin tones result in less production of vitamin D than lighter skin tones. In addition, age, geographic location, time of day, and calendar season all affect the skin production of vitamin D.
Dietary sources of vitamin D are limited to fortified dairy products and fatty fish. Therefore, the use of a supplement containing vitamin D is the most practical means of addressing vitamin D sufficiency. A high prevalence of vitamin D insufficiency has been found in young adults with seemingly adequate sun exposure living at latitude 21°, as well as in postmenopausal women receiving treatment for osteoporosis living in all regions of the continental United States.[117,101]Women who are older, frail, chronically ill, housebound, or institutionalized, or those who live in northern latitudes are particularly at risk for vitamin D deficiency.[104] Vitamin D supplementation of at least 800 to 1,000 IU/day thus appears to be appropriate year-round for all women. The NAS has established the upper limit of safe intake for vitamin D as 2,000 IU/day.[106] However, many authorities consider this amount to be overly conservative.[118] Doses greater than 10,000 IU/day may be associated with risks of hypercalciuria and hypercalcemia.
At present, there is some controversy as to whether the preferred over-the-counter vitamin D supplement is vitamin D3(cholecalciferol).[119–121] Vitamin D3 or vitamin D2 (ergocalciferol) is found in various over-the-counter products, although vitamin D3 has become increasingly common. The only prescription form of vitamin D currently is vitamin D2. Most multivitamins contain a minimum of 400 IU of vitamin D per tablet, although recent formulations of multivitamins directed toward women may contain as much as 800 IU.
Many calcium supplements are combined with vitamin D. The vitamin D contained in such combination calcium supplements or multivitamins is considered a prehormone for the active form of vitamin D, 1,25-dihydroxyvitamin D, which is ultimately produced in the kidney. Thus, the consumption of calcium and vitamin D2 or D3 at the same time is not relevant to the absorption of the calcium just consumed, but this is a convenient combination.
Consensus expert opinion is that levels of serum 25(OH)D that are indicative of vitamin D sufficiency in the context of bone health are minimally 20 ng/ml (50 nmol/L) with the majority favoring 29–32 ng/ml (70–80 nmol/L).[113,114] These levels for serum 25(OH)D were chosen primarily on the basis of studies indicating that PTH levels are lowest at serum 25(OH)D levels of 28 to 45 ng/ml (70–110 nmol/L) and that calcium absorption efficiency plateaus at concentrations of serum 25(OH)D at or above approximately 32 ng/ml (80 nmol/L).[122] There appears to be no justification for attempting to increase serum 25(OH)D levels above 60 ng/ml (150 nmol/L).[117]
As a rough guide, the serum 25(OH)D level, under steady-state dosing, will rise by about 1 nmol/L per μg cholecalciferol/day. Thus, an individual with a serum 25(OH)D value of 20 ng/mL (50 nmol/L) will typically need at least 30 μg of additional vitamin D3/day (1,200 IU) to reach a level of 32 ng/mL (80 nmol/L)—or, to use the units commonly reported in the United States, serum 25(OH)D will rise by about 1 ng/mL for every 100 IU/day of additional cholecalciferol.[121,122] In measuring serum 25(OH)D, it is important to recognize that a new steady state is not achieved before 3 months on a new dose of vitamin D. In addition, not all assays for 25(OH)D may capture 25(OH)D2 as well as 25(OH)D3. This is extremely relevant if vitamin D2 is used as a supplement instead of vitamin D3 or if high-dose prescription vitamin D2 (eg, 50,000 IU/wk for 8 wk) is being used for quick repletion in an individual with vitamin D deficiency.
Various studies have shown that 60% to nearly 100% of individuals—whether institutionalized or free-living, or whether using vitamin D supplements or not—have serum 25(OH)D values below 32 ng/mL (80 nmol/L). The NOF recommends the measurement of 25(OH)D in patients at risk for vitamin D deficiency.[104] However, the high prevalence of vitamin D deficiency, a treatable cause of bone loss, is part of the rationale for a more general recommendation to measure the 25(OH)D level in patients with low bone mass. It is also important to note that the 1,25-dihydroxyvitamin D level is not the appropriate measurement to assess vitamin D stores. Vitamin D deficiency is nearly universal among individuals over age 90.[122]
The effect of vitamin D alone on fracture risk is becoming clearer, appearing to depend heavily on both compliance and dose. Several large trials evaluating the effect of vitamin D in doses ranging from 400 IU to 800 IU/day combined with 1,000 mg of elemental calcium failed to show a fracture risk reduction benefit.[98,123] However, a meta-analysis[124] of 12 randomized clinical trials in postmenopausal women (mean ages, 71–85 y) found that the higher vitamin D dose of 700 to 800 IU/day was associated with significant reductions in the risk of both hip and nonvertebral fractures, whereas no risk reduction was seen in trials or cohorts using a dose of 400 IU vitamin D. In the WHI,[98] although no reduction in hip fracture risk from vitamin D and calcium supplementation was seen in the entire cohort, when the analysis was restricted to adherent women, there was a significant reduction in hip fracture risk with 400 IU of vitamin D and 1,000 mg of elemental calcium per day.
Studies have found that vitamin D (600–700 IU/d) with supplemental calcium can reduce the rate of postmenopausal bone loss, especially in older women.[125] Results from the WHI[98] found calcium (1,000 mg/d) plus vitamin D (400 IU/d) recipients had a small but significant 1% improvement in hip BMD. Vitamin D supplementation also has been found to improve muscle strength[126] and balance,[127,128] and reduce the risk of falling.[129]
Vitamin K The current adequate intake value for vitamin K is 90 μg/day.[130] The predominant form of vitamin K is vitamin K1 (phylloquinone), found in green leafy vegetables, although the bioavailability of this form of vitamin K is not assumed to be more than 20%. Approximately 34% of vitamin K is obtained from fats and oils in the North American diet. The average dietary intake of vitamin K is approximately 340 μg/day. In one study, supplementation with vitamin K1 (1 mg/d) in conjunction with calcium, magnesium, zinc, and vitamin D appeared to be associated with beneficial effects on bone turnover and bone density at the femoral neck.[131] Another study, in which 2 mg/day of vitamin K1 was given in conjunction with calcium and vitamin D, suggested a beneficial effect on bone density at the ultradistal radius but not at the femoral neck or trochanter.[132] A third study suggested no benefit to 5 mg/day of vitamin K1 in preventing bone loss at the lumbar spine and proximal femur for postmenopausal women with adequate vitamin D intake who have osteopenia.[133]
There are no known adverse effects from high doses of vitamin K in otherwise healthy women, but strong evidence that vitamin K1 is useful in the prevention or treatment of postmenopausal osteoporosis is lacking. Vitamin K supplements are contraindicated in women taking warfarin.
Magnesium Another nutrient, magnesium, is sometimes mentioned as a necessary supplement for the protection of bone health and/or for absorption of calcium. The RDA for magnesium is 320 mg/day in women age 31 and older. Magnesium is plentiful in foods.[106] Green leafy vegetables, unpolished grains, and nuts are rich in magnesium. Despite this, dietary intake of magnesium is generally below the RDA, reported as a mean intake of 258 mg/day in women ages 40 to 59 and 236 mg/day in women age 60 and older.[97] The total intake of magnesium is generally dependent on the total caloric intake; magnesium intake tends to fall after age 70. Severe magnesium deficiency, as seen in advanced malnutrition from any cause, can result in hypocalcemia and resistance to vitamin D. Data supporting a role for magnesium supplementation in the prevention or treatment of postmenopausal osteoporosis, however, are inconclusive.[134–136] Magnesium supplementation does not appear to enhance or inhibit calcium absorption.[137] In women with excessive magnesium loss (usually due to GI disease [eg, diarrhea, vomiting], loop diuretics, or chemotherapy), magnesium supplementation would be appropriate.[138,139]
Protein For women older than age 75, data from the Framingham Osteoporosis Study, a longitudinal cohort study, suggest that adequate protein intake may help minimize bone loss.[140,141] Protein supplements (20 g/day) in older patients (mean age, 82 y) who have sustained a hip fracture have been shown to significantly shorten the hospital stay (median stay, 69 d vs 102 d for placebo recipients) after hip fracture and improve the clinical outcomes while in the hospital.[142] Compared with the controls, protein recipients also had significantly lower rates of complications and mortality 7 months after their hip fracture.
Concerns have been raised in the past that high protein intake may result in increased urinary calcium excretion and increased acid production, both detrimental to bone health. However, a negative calcium balance is likely to result only if the daily calcium intake is inadequate. The negative effect of acidity on the skeleton from dietary protein is relatively minor. Rather than reducing protein intake, a more appropriate measure would be to increase dietary intake of fruits and vegetables for their alkalizing effect.[143] Dietary protein overall is positively linked to the maintenance of bone and muscle health. Therefore, some experts suggest that the current recommended intake of protein may be inadequate for optimum skeletal and muscle health.[143]
Isoflavones Isoflavones are a class of phytoestrogens found in rich supply in soybeans, soy products, and red clover.[144]These are diphenolic compounds with structural similarities to estrogen. The dietary phytoestrogens of primary interest found in soybeans are genistein, daidzein, and glycitein. Ipriflavone, a synthetic isoflavone available without a prescription in the United States and Canada, has not demonstrated a positive effect on bone density, bone turnover markers, or fracture risk in women with osteoporosis.[145]
Data suggesting any benefit of dietary isoflavones in the prevention or treatment of postmenopausal osteoporosis, regardless of the source, are relatively weak.[146–148] Benefits, in terms of bone density and turnover, are minor at best. In a recent study from Italy, 2 years of purified genistein in a dose of 54 mg/day resulted in small but statistically significant increases in BMD at the lumbar spine and femoral neck compared with placebo.[149] Genistein was provided as a tablet, however, and not as part of the diet, and GI side effects resulted in 19% of the genistein-treated women discontinuing the study. Other studies suggest no benefit whatsoever in the prevention or treatment of postmenopausal osteoporosis.[149–153] A meta-analysis of RCTs studying the overall effect of soy isoflavones on BMD concluded that soy isoflavone supplementation was unlikely to have a significant favorable effect on BMD.[154]
Exercise
Weight-bearing and strength-training exercises are beneficial to bone development and maintenance.[155–157] Local increases in bone mass occur in response to activities that cause major stress to bone. The most dramatic example is a comparison of the BMD in the dominant and nondominant arms of tennis players, in which the BMD in the dominant arm is markedly greater.[158] Extreme exercise is not necessary, however, to effect a bone benefit. Even mild forms of exercise that improve agility and balance can benefit the skeleton. Active weight-bearing or strength-training exercises can increase bone mass if they increase muscle mass and strength. Applying passive stress to bone also shows promise, with the most positive results coming from use of high-frequency, whole-body vibration systems.[159,160]
Weight-bearing exercise can be as simple as brisk walking. Jogging or running provides impact-loading benefits to the skeleton. In early postmenopausal women, strength-training provides small but significant benefits to bone mass.[161] A meta-analysis[162] found that postmenopausal women who exercised increased their spinal BMD by approximately 2%. For estrogen-replete women who use ET, strength training provides additional BMD benefits over therapy alone.[163] Most strength-training studies have used progressive resistance obtained with machines designed for this purpose (eg, Nautilus). However, strength training need not involve expensive equipment. Resistance bands, free weights, or barbells can be used in place of resistance machines. Strength training or resistance exercises target specific muscle groups. It is necessary to target the large extensor muscles of the back, the hip flexors and extensors, muscles of the thigh, upper arm, and forearm in order to affect areas of the skeleton most often involved in osteoporotic fractures.
Exercise for women with osteoporosis should not include high-impact aerobics or activities in which a fall is likely, such as exercising on slippery floors or step aerobics. Activities requiring repeated or resisted trunk flexion, such as sit-ups or toe touches, should also be avoided because of the increased loads placed on the spine during such activities that may result in spine fracture. It is nevertheless important that osteoporotic women remain as physically active as possible. Physical activity plays an important role in reducing the risk of falls by maintaining muscle strength, agility, and balance. Among women age 75 and older, muscle strengthening and balance exercises have been shown to reduce the risk of falls and fall-related injuries by 75%.[164]
Even women who are severely physically impaired can generally perform water aerobics with no impact on the skeleton to maintain muscle strength and balance. Gentle spinal extension exercises can also be performed while seated, helping to strengthen the back extensors and lift the lower ribs off the pelvis. Exercises to strengthen back extensor muscles have been shown to reduce the risk of spine fracture, both in women without prior fracture[165] and in women with prior fracture who had undergone percutaneous vertebroplasty,[166] as well as to improve quality of life.[167]
Fall Prevention
Falls are the precipitating factor in nearly 90% of all appendicular fractures, including hip fracture.[168] In the United States and Canada, approximately one third of women over age 60 fall at least once a year.[80,169] In nearly one half of these cases, it is a recurrent fall. The incidence of falls increases with age, rising to a 50% annual rate in people over age 80. Older women have a significantly higher risk for falls than do men of the same age. Theoretically, the intervention that may reduce appendicular fracture risk most rapidly is fall prevention. As a result, prevention of falls should be an aspect of routine care for all postmenopausal women.
Several healthcare interventions have proven effective in reducing the risk of falls. These focus primarily on exercises to improve balance and muscle strength, adjusting medication use (especially psychotropic drugs), and reducing fall hazards in the home.[170] Tapering or discontinuing use of benzodiazepines, neuroleptic agents, and antidepressants has been found to reduce the risk of falling by more than 60%.[171] Implementing relatively inexpensive measures to eliminate safety hazards in the home may also reduce this risk (see Table 5), but home hazard intervention studies have failed to show significant reductions in fracture.[170]
Table 5. Recommendations for fall prevention
| Lighting |
| Provide ample lighting |
| Have easy-to-locate light switches for rooms and stairs |
| Use night-lights to illuminate pathways from bedroom to bathroom and kitchen |
| Provide light on all stairways |
| Obstructions |
| Remove clutter, low-lying objects |
| Remove raised door sills to ensure smooth transition |
| Floors and carpets |
| Provide nonskid rugs on slippery floors |
| Repair/replace worn, buckled, or curled carpet |
| Use nonskid floor wax |
| Furniture |
| Arrange furniture to ensure clear pathways |
| Remove or avoid low chairs and armless chairs |
| Adjust bed height if too high or low |
| Storage |
| Install shelves and cupboards at accessible height |
| Keep frequently used items at waist height |
| Bathroom |
| Install grab bars in tub, shower, near toilet |
| Use chair in shower and tub |
| Install nonskid strips/decals in tub/shower |
| Elevate low toilet seat or install safety frame |
| Stairways and halls |
| Install handrails on both sides of stairs |
| Remove or tape down throw rugs and runners |
| Repair loose and broken steps |
| Install nonskid treads on steps |
Hip protectors worn during the day have been shown to reduce the likelihood of hip and pelvis fractures from falls among older postmenopausal women (≥75 y) with a history of frequent falls although they obviously do not reduce the risk of falling itself.[172] However, a Cochrane review[173] found the overall evidence inconclusive regarding efficacy in reducing hip fractures. Furthermore, the adherence rates in studies were low, averaging approximately 50%, primarily due to the inconvenience of wearing the protective garment day and night.
Smoking Cessation
Compared with nonsmokers, women smokers tend to lose bone more rapidly, have lower bone mass, and reach menopause 2 years earlier, on average.[174–176] In addition, some data show that postmenopausal women who currently smoke have significantly higher fracture rates than nonsmokers.[177] The risk imparted by smoking remains significant even after adjusting for BMD.[178]
The mechanisms by which smoking might adversely affect bone mass are not known, although evidence suggests that cigarette smokers may have impaired calcium absorption[174,179,180] and lower 17β-estradiol levels.[181]
Meta-analyses have also suggested the risk of hip fracture may be markedly increased in current smokers.[182] In current smokers, the risk of hip fracture is similar in women up to age 50, but then increases with age with a risk ratio of 1.17 at age 60, increasing to 1.71 at age 80. The RR is only modestly adjusted downward when corrected for BMD. Current smoking is associated with significantly increased risk of any fracture, any osteoporotic fracture, and hip fracture in women.[33] The mechanism is unclear: it may be related to lower levels of activity, morbidity, risk of falls, or changes in microarchitecture.[33]The WHO findings indicate that a history of smoking confers a substantial risk for future fracture, largely independent of BMD.[178]
Smoking cessation and avoidance of secondhand smoke for nonsmokers is important as a general health measure because of the numerous health problems associated with smoking. Lower BMD and increased fracture risk are two of these health problems.[156,178] A wide array of smoking cessation aids are available, including prescription products (with and without nicotine) and behavior-modification programs.
Alcohol Consumption
Data suggest an association between moderate alcohol intake and increased BMD in postmenopausal women.[183,184]Nevertheless, this observation must be tempered by the increased risk of falling and osteoporotic fracture associated with alcohol consumption. The level of alcohol consumption associated with an increased risk of falls is more than seven units a week, as established by the Framingham Heart Study.[185] Two or more units of alcohol within 6 hours is estimated to account for approximately 20% of falls at home among working-age adults.[186] Data from more than 11,000 women from three different cohorts suggest that alcohol consumption of more than two units a day is associated with an increased risk of osteoporotic fracture.[187] Therefore, postmenopausal women who drink should be advised to drink moderately and not exceed seven units of alcohol a week, with preferably no more than two in any one 6-hour period. One unit is considered to be 12 oz (360 mL) of beer, 4 oz (120 mL) of wine, or 1 oz (30 mL) of liquor.
Management: Pharmacologic Approaches
A management strategy focused on lifestyle approaches may be all that is needed for postmenopausal women who are at low risk for osteoporotic fracture. NAMS recommends adding osteoporosis drug therapy in the following populations:
- All postmenopausal women who have had an osteoporotic vertebral or hip fracture
- All postmenopausal women who have BMD values consistent with osteoporosis (ie, T-scores equal to or worse than −2.5) at the lumbar spine, femoral neck, or total hip region
- All postmenopausal women who have T-scores from −1.0 to −2.5 and a 10-year risk, based on the FRAX calculator, of major osteoporotic fracture (spine, hip, shoulder, or wrist) of at least 20% or of hip fracture of at least 3%
Several pharmacologic options are available for osteoporosis therapy, including bisphosphonates, the selective estrogen-receptor modulator (SERM; also known as estrogen agonist/antagonist) raloxifene, PTH, estrogens, and calcitonin. No studies have prospectively compared these therapies for antifracture efficacy.
With the exception of estrogen, the effects of therapies on fracture have been demonstrated only in patients with either the clinical or BMD diagnosis of osteoporosis. The absolute reduction in fracture risk is greatest in patients at high risk of fracture.
Adherence to therapy is poor. In studies of 6 months to 1 year, adherence rates for prescription drugs ranged from below 25% to 81%, depending on the therapy.[188–190] Perhaps the most important follow-up measure for clinicians is to encourage adherence to the treatment plan and to identify barriers to nonadherence. Providing clear information to women regarding their risk for fracture and the purpose of osteoporosis therapy may be the optimal way to improve adherence.
Bisphosphonates
This class of drugs works by inhibiting the activity of osteoclasts and shortening their lifespan, thereby reducing bone resorption.[191] Bisphosphonates do not have known beneficial effects on the body other than on bone. The most common adverse effect of oral bisphosphonate therapy is esophageal and gastric irritation, particularly affecting individuals who dose inappropriately. Before starting bisphosphonate therapy, patients should be screened for secondary causes of low bone mass. Those with low serum calcium should not receive bisphosphonates. Serum creatinine should be used to estimate the glomerular filtration rate; treatment may be initiated only if the rate is 30 mL/min or greater (≥35 mL/min with IV zoledronic acid).
Clinical trials have demonstrated that bisphosphonates significantly increase BMD at the spine and hip in a dose-dependent manner in both younger and older postmenopausal women. In women with osteoporosis, bisphosphonates have reduced the risk of vertebral fractures by 40% to 70% and reduced the incidence of nonvertebral fracture, including hip fracture, by about half this amount.[88,191]
Most of the bisphosphonates approved for osteoporosis therapy in both the United States (alendronate, ibandronate, and risedronate) and Canada (alendronate, etidronate, and risedronate) are available in oral formulations for daily and intermittent dosing regimens. Zoledronic acid is available only as an IV injection. Weekly oral dosing regimens of alendronate and risedronate, monthly oral dosing regimens of ibandronate and risedronate, and IV dosing of ibandronate every 3 months have been approved based on clinical trials that showed BMD responses equivalent to those observed with daily treatment.[192–195] All fracture data with alendronate, ibandronate, and risedronate are from trials with daily dosing; the bridging studies beyond daily dosing were not designed with fracture end points. The fracture data with zoledronic acid are from the study with annual IV dosing.
Alendronate This bisphosphonate, marketed as Fosamax, is approved as an oral tablet in both the United States and Canada for postmenopausal osteoporosis prevention (5 mg/d or 35 mg/wk) and treatment (10 mg/d or 70 mg/wk). Alendronate is also available in a single weekly oral tablet of 70 mg with 5,600 IU of vitamin D (Fosamax Plus D; Fosavance). Several generic preparations of alendronate are available in both Canada and the United States. These preparations are less validated and may have tolerability and absorption differences from the branded product.
For women in early postmenopause, 2 to 6 years of treatment with alendronate (≥5 mg/d) has been shown to significantly increase BMD at the spine and hip by approximately 1% to 4% from baseline, whereas BMD in placebo recipients decreased by 2% to 4% during that time.[196,197] In older women with osteoporosis,[198] therapy with 10 mg/day significantly increased BMD in the spine (8.8%) and the femoral neck (5.9%) after 3 years, compared with placebo. In 7- and 10-year extension trials in women with low bone density,[199,200] alendronate therapy resulted in increases from baseline of 5% to 10% at the spine and hip in postmenopausal women who had low BMD or established osteoporosis. Because placebo groups were not followed for the duration of the studies,[199,200] the antifracture effects of long-term alendronate therapy could not be adequately evaluated. However, there was no apparent increase in fracture risk over time.
The efficacy of alendronate in decreasing fracture risk has been demonstrated only in postmenopausal women with osteoporosis. Similar to other bisphosphonates, alendronate has shown lesser effects in women without osteoporosis.
In the Fracture Intervention Trial (FIT),[201] daily alendronate therapy for 2.9 years significantly reduced the risk of vertebral fracture by 47% and of hip fracture by 51% in women with low BMD and previous vertebral fracture. The incidence of clinical vertebral fractures was reduced by 59% within the first year.[202] In a composite analysis of the two arms of the FIT study,[202] 3 years of alendronate therapy in a subgroup of women with osteoporosis (ie, vertebral fracture or T-score equal to or worse than −2.5) significantly reduced the risk of nonspine fracture by 27% and new spine fracture by 50%.
Risedronate This bisphosphonate, marketed as Actonel, is approved in the United States and Canada for the prevention and treatment of postmenopausal osteoporosis in oral tablet doses of 5 mg/day, 35 mg/week, 75 mg on 2 consecutive days once a month, and 150 mg/month.
In an RCT of early postmenopausal women (age range, 40–61 y; mean age, 51–52 y) with normal bone density, risedronate doses of 5 mg/day for 2 years produced significant BMD increases of 5.7% in the lumbar spine and 5.4% in the hip greater than with placebo.[203] In an RCT in older postmenopausal women (mean age, 68–69 y),[39] 3 years of risedronate therapy (5 mg/d) resulted in significant BMD increases of 4.3% in the spine and 2.8% in the femoral neck compared with placebo. Therapy for 7 years resulted in progressive increases in BMD of 11.5% from baseline (with no placebo group after 5 y).[204]
Several RCTs have found fracture risk reductions with risedronate. In two trials of postmenopausal women with osteoporosis,[39,205] 3 years of treatment with 5 mg/day of risedronate significantly reduced the risk of vertebral fracture (by 41%–49%) compared with placebo. Within the first year of therapy, the RR of vertebral fracture was reduced by 61% to 65%. After 3 years of therapy, vertebral fracture risk reductions were still statistically significant relative to placebo. In one of these trials,[37] the risk of nonvertebral fracture was significantly reduced by 39%. In the other trial,[205] nonvertebral fracture risk was reduced by 33%, although this was not statistically significant versus placebo.
In the Hip Intervention Program Study Group,[206] an RCT of 5,445 postmenopausal women ages 70 to 79, daily risedronate therapy reduced the RR for hip fracture by 40% in women with BMD values consistent with osteoporosis. In a post hoc analysis, risedronate reduced the risk of hip fracture by 60% in the group with prior vertebral fractures. However, therapy did not markedly lower the hip fracture risk in women age 80 and older who had risk factors for fracture but who did not have BMD testing performed to confirm osteoporosis.
In an RCT of 265 postmenopausal women (mean age, 72 y), the incidence of vertebral fractures in women treated with risedronate 5 mg/day was significantly reduced during years 4 and 5 compared with placebo,[207] and appeared to remain reduced through 7 years of treatment (no placebo group after 5 years).[204] No new adverse events were observed in these trials.
Ibandronate Ibandronate, marketed as Boniva, is approved as a 2.5-mg oral tablet once a day, as well as a 150-mg tablet once a month for the prevention and treatment of postmenopausal osteoporosis. It is also approved in an IV formulation at a 3-mg dose every 3 months (administered by a healthcare professional) for the treatment of postmenopausal osteoporosis.
In early postmenopausal women (mean ages, 57.6–58.8 y) without osteoporosis, those receiving oral ibandronate at 2.5 mg/day had significant BMD increases of 1.9% in the lumbar spine (vs −0.9% for placebo) and 1.2% in the total hip (vs −0.6% for placebo) after 2 years.[208] In older women (mean age, 69 y) with low spinal BMD and current vertebral fractures, oral ibandronate at 2.5 mg/day significantly increased BMD compared with placebo in the spine (5.2%) and femoral neck (4.1%) after 3 years.[209] Daily oral ibandronate therapy reduced morphometric vertebral fractures by 52% over 3 years, but there was no important effect on nonvertebral fracture risk in the overall study population. In a post hoc analysis, a 69% reduction of nonvertebral fracture risk was described, but only in the subgroup of study patients with baseline femoral neck T-scores below 3.
Zoledronic Acid The bisphosphonate zoledronic acid, marketed as Reclast in the United States and Aclasta in Canada, is approved for osteoporosis treatment in postmenopausal women. The annual 5-mg IV infusion is administered by a healthcare professional over a period of no less than 15 minutes. An infusion administered once every 2 years is now approved in the United States for prevention of osteoporosis in postmenopausal women.
In an RCT of 7,765 postmenopausal women with osteoporosis (mean age, 73 y), zoledronic acid in an IV dose of 5 mg given once yearly for 3 years produced significant BMD increases of 6.7% in the lumbar spine and 6.0% in the hip greater than with placebo.[210] Vertebral fracture risk was reduced by 70%, hip fracture risk by 41%, and nonvertebral fracture risk by 25%. In a separate study of 2,127 women and men with a recent osteoporotic hip fracture who had received postfracture treatment with vitamin D, annual IV dosing of 5-mg zoledronic acid reduced the incidence of clinical fracture by 35% and of all-cause mortality by 28%.
Etidronate The bisphosphonate etidronate, marketed as Didrocal oral tablets, is approved in Canada for osteoporosis prevention and treatment in postmenopausal women (400 mg/d for 14 d every 3 months, with calcium taken between cycles). In the United States, etidronate is approved only for treatment of Paget's disease, not for osteoporosis therapy.
There have been no controlled trials demonstrating fracture risk reduction with cyclic etidronate therapy. A meta-analysis[211]of 13 trials investigating intermittent cyclic etidronate therapy for postmenopausal osteoporosis found that, relative to control groups, 1 to 3 years of therapy increased BMD by 4.1% in the lumbar spine and 2.3% in the femoral neck. This analysis concluded that etidronate significantly reduced the risk for vertebral fracture (37%) but not the risk for nonvertebral fracture.
For osteoporosis therapy, etidronate is typically administered at 400 mg/day for 14 days every 3 months. Dosing, as with other bisphosphonates, is best on an empty stomach before breakfast with only a glass of water. Calcium and vitamin D must be continued as detailed above. A cyclic regimen is used because daily high-dose use may interfere with bone mineralization.[212] This is not the schedule for Paget's disease.
Adverse Events with Bisphosphonate Therapy Oral bisphosphonates may cause upper GI disorders such as dysphagia, esophagitis, and esophageal and gastric ulcer, a contraindication in those with esophageal abnormalities that delay esophageal emptying or in those who are unable to stand or sit upright for at least 30 to 60 minutes after ingestion. Studies are not adequate to determine upper GI adverse-event differences among oral bisphosphonates. Neither IV ibandronate nor IV zoledronic acid has been associated with upper GI adverse events.
All bisphosphonates carry precautions on hypocalcemia and renal impairment. Serum calcium and serum creatinine should be measured in all patients before beginning osteoporosis therapy. Although no cases of acute renal failure have been observed in clinical trials, patients who receive IV ibandronate or zoledronic acid should have serum creatinine measured before administration of each dose.
Oral bisphosphonates are poorly absorbed; typically, approximately 0.5% of an oral dose is absorbed, even when taken on an empty stomach with plain water. Therefore, oral bisphosphonates must be taken the first thing in the morning when the stomach is empty. Food, drink, and medications (including supplements) must be avoided for 30 minutes (alendronate and risedronate) to 60 minutes (ibandronate) after dosing; etidronate labeling recommends waiting 2 hours.
A transient flu-like illness, often called an acute-phase reaction, occurs infrequently with large doses of oral or IV bisphosphonates. This has been observed infrequently after monthly oral dosing with ibandronate and risedronate and more commonly with IV dosing with ibandronate and zoledronic acid. Symptoms are generally mild, most often occurring with the first but not subsequent doses, and are treated symptomatically.
A theoretical concern exists regarding possible oversuppression of bone turnover with long-term bisphosphonate therapy, resulting in a more brittle skeleton. Individual cases and small case series of patients with unusual, poorly healing fractures of the subtrochanteric region of the femur have been described in patients receiving bisphosphonates.[213–215] It is unclear whether these unusual fractures are the result of treatment or a consequence of their underlying osteoporosis.
Jaw lesions, usually after dental extraction (known as osteonecrosis of the jaw; ONJ), have been observed with bisphosphonate use, most often in patients treated with large IV doses for cancer-related bone diseases.[216,217] ONJ has been defined as a delay in healing of an oral lesion after surgery or extraction for more than 6 to 8 weeks. Cases have also been reported in patients receiving bisphosphonate therapy for osteoporosis.[218,219] The incidence of these lesions is not known, and a causal association between bisphosphonates and osteonecrosis has not been documented. There are no data to recommend the discontinuation of bisphosphonate therapy before dental extraction (although therapy may be suspended until the oral lesion has healed). There are no data to suggest that dental surgery is contraindicated in patients on bisphosphonate therapy. Routine dental care is recommended for all patients.
Long-term Safety of Bisphosphonate Therapy RCTs of more than 5 years' duration with alendronate or risedronate[197,199,200,204] have demonstrated persistent but not progressive reduction of bone turnover without evidence of unexpected adverse effects or abnormal bone histomorphometry. Smaller numbers of patients have been followed for 7 years on risedronate and for 10 years on alendronate. No data are available on effects of long-term (>3 y) ibandronate or zoledronic acid therapy. Current evidence does not support recommendations regarding the optimal duration of bisphosphonate therapy.
Discontinuation of Bisphosphonate Therapy After discontinuation of alendronate after 5 years of therapy, BMD remains stable or decreases slowly while bone turnover markers remain below baseline values for up to 5 years.[199,200,220] Whether the fracture protection afforded by alendronate therapy persists after discontinuation is not known. In one study,[210] the incidence of nonvertebral fractures was similar in patients who stopped and in those who continued therapy after being on alendronate for an average of 5 years. However, the incidence of painful vertebral fractures was significantly greater in those patients who discontinued therapy. In a review of a large medical claims database, patients who discontinued alendronate therapy after 2 years had an increased rate of hip fracture compared with patients who continued treatment.[221]
Discontinuation of risedronate therapy after 2 years in young postmenopausal women (mean ages, 51–52 y) has been shown to result in significant bone loss at both the spine and hip during the first year after treatment is stopped.[203] In older women with osteoporosis, discontinuation after 3 years was associated within 12 months with bone loss and return of bone markers to levels in the placebo group.[222] Vertebral fracture risk remained reduced during the 12 months after discontinuation of treatment in those patients who had taken risedronate.
No data are available regarding discontinuation of etidronate, ibandronate, or zoledronic acid therapy.
Selective Estrogen-receptor Modulators
These nonsteroidal agents of various chemical structures act as estrogen agonists and/or antagonists. The SERM raloxifene (marketed as Evista oral tablets) is government approved for the prevention and treatment of osteoporosis at a dose of 60 mg/day. No other SERM is approved for osteoporosis therapy, although several are in clinical development. (See also section on "Promising new therapies.")
Raloxifene has beneficial effects on BMD, and it decreases bone turnover as assessed by biochemical markers. In a 2-year RCT of 601 postmenopausal women without osteoporosis (mean age, 55 y), raloxifene at a dose of 60 mg/day significantly improved BMD at the lumbar spine (1.6%) and femoral neck (1.2%) compared with placebo (decreases of 0.8% and 1.2%, respectively).[223] In the RCT Multiple Outcomes of Raloxifene Evaluation (MORE) trial evaluating postmenopausal women with osteoporosis (mean age, 67 y),[224] 3 years of raloxifene therapy at 60 mg/day significantly increased BMD versus placebo by 2.6% at the spine and 2.1% at the femoral neck.
The efficacy of raloxifene in reducing osteoporotic fractures was also demonstrated in the MORE trial.[224] After 3 years of therapy, 60 mg/day raloxifene reduced the risk of vertebral fracture by 55% in women with a femoral neck or lumbar spine BMD T-score of −2.5 or below and by 30% in women with low T-scores and an existing vertebral fracture; both findings were significant compared with placebo. A 1-year blinded extension of the MORE trial[225] found persistent vertebral fracture risk reductions of 50% and 38% in the two groups, respectively. A separate analysis revealed that at 1 year, raloxifene (60 mg/d) reduced the risk of new clinical vertebral fracture by 68% in the overall study population.[226] No raloxifene effect has been observed on hip or other nonvertebral fracture risk.
In addition to its effects on bone, raloxifene has been associated with a reduced risk of invasive breast cancer in postmenopausal women with osteoporosis. In the MORE trial, the overall incidence of invasive breast cancer was significantly reduced by 76% after 3 years[227] and 72% after 4 years.[228] In a 4-year extension of the MORE trial—the Continuing Outcomes Relevant to Evista (CORE) trial[229]—the risk after 8 years was 59% lower in raloxifene recipients; the risk of estrogen receptor (ER)-positive invasive breast cancer was 66% lower. The combined results show invasive breast cancer and ER-positive breast cancer risks were reduced by 66% and 76%, respectively. It should be noted that the MORE-CORE studies were conducted on postmenopausal women initially selected for risk of osteoporosis, not for risk of breast cancer. In the United States but not Canada, raloxifene is indicated for the prevention of breast cancer in women at high risk.
A significant increase in venous thromboembolic (VTE) events was noted in the MORE trial.[230] However, a secondary analysis of the MORE trial data[231] found no overall significant differences in the number of coronary or cerebrovascular events between placebo and raloxifene, although in a subset of women with increased cardiovascular risk at baseline, raloxifene significantly reduced cardiovascular risk. Again, it should be noted that the MORE trial was not designed with cardiovascular outcomes as a primary objective.
In the MORE-CORE trial, women defined as at increased cardiovascular risk had neither a beneficial nor a harmful effect of raloxifene,[232] similar to the findings in Raloxifene Use for the Heart (RUTH).[233] The rare risk of fatal stroke reported in RUTH appears to be confined to women at baseline increased risk of stroke (Framingham Stroke Risk Score ≥13).[234] When selecting women for raloxifene therapy, consider baseline cerebrovascular risk.
RCTs of more than 5 years' duration in women with osteoporosis have demonstrated no other significant adverse effects.[230]Raloxifene therapy may be associated with an increase in vasomotor symptoms and leg cramps. However, it does not increase the risk of cataracts, gallbladder disease, endometrial hyperplasia, or endometrial cancer, or cause vaginal bleeding or breast pain.[224,230]
Bone loss often resumes when raloxifene therapy is stopped.[235,236]
Parathyroid Hormone
PTH or its analogues, given by subcutaneous injection once daily, are anabolic agents that directly stimulate osteoblastic bone formation, resulting in substantial increases in trabecular bone density and connectivity in women with postmenopausal osteoporosis. This mechanism of action is very different from that of antiresorptive agents such as estrogen and bisphosphonates, which reduce bone resorption.
Teriparatide (recombinant human PTH 1–34), marketed as Forteo, is approved in both the United States and Canada for the treatment of osteoporosis in postmenopausal women who are at high risk for fracture. In RCTs, daily subcutaneous injections of teriparatide stimulated bone formation and improved bone density in postmenopausal women, regardless of whether they were receiving ET.[237–239] In postmenopausal women with prior vertebral fracture,[239] 19 months of teriparatide treatment (20 µg/d) significantly increased bone density in the spine by 8.6% and in the femoral neck by 3.5% compared with placebo. The incidence of new vertebral fractures was reduced by 65% and new nonvertebral fragility fractures by 53%, although the study was not designed to examine the effect on hip fractures. Teriparatide is also indicated for the treatment of glucocorticoid-induced osteoporosis and male osteoporosis.
Drug-related adverse effects include muscle cramps and infrequent hypercalcemia, nausea, and dizziness. High-dose teriparatide treatment has caused bone tumors (osteosarcoma) in a rat model at doses ranging from 3 to 60 times the 20 µg/day dose in humans,[240] although the significance of this finding in humans is uncertain. Teriparatide should not be administered to postmenopausal women with hypercalcemia, bone metastases, disorders that predispose them to bone tumors such as Paget's disease, or those who received prior skeletal irradiation. Forteo is indicated for no longer than 24 months in the United States and for no longer than 18 months in Canada.
When PTH therapy has been stopped, substantial bone loss has occurred within the first year.[241] However, in RCTs using PTH 1–84, administering alendronate after discontinuing PTH therapy was shown to maintain or improve BMD,[241,243]although prior alendronate treatment tends to slow bone turnover and delay the PTH-induced increases in BMD and bone turnover response by 3 to 6 months.[243] It is unclear whether a second course of PTH can be safely restarted after a period without therapy or whether regimens other than daily can be effective. A recommendation can be made for treatment with antiresorptive therapy following a course of PTH. (See also the section on "Promising new therapies.")
Estrogens
Systemic estrogen products (estrogen plus progestogen [EPT] for women with a uterus or ET for women without a uterus) are government approved in the United States and Canada for prevention, but not treatment, of postmenopausal osteoporosis. A number of RCTs have evaluated the effect of systemic estrogen on BMD and fracture in postmenopausal women.
BMD The beneficial effects of systemic oral or transdermal ET/EPT at standard doses on BMD preservation are well established. A 2002 meta-analysis[244] of 57 RCTs comparing ET/EPT against placebo in postmenopausal women found consistent BMD increases with ET/EPT at all sites. In trials of 2 years duration, the mean difference in BMD after ET/EPT was 6.8% at the lumbar spine and 4.1% at the femoral neck.
The two largest and best controlled trials support these findings. In the Postmenopausal Estrogen/Progestin Interventions trial[245] (N = 875), standard daily doses of 0.625 mg conjugated estrogens (CE), with or without a progestogen (either MPA or micronized progesterone), for 3 years significantly increased spinal BMD by 3.5% to 5.0%, with a 1.7% increase in hip BMD. The WHI,[246] a 5-year RCT in postmenopausal women ages 50 to 79 (N = 16,608), reported that standard doses of daily EPT (0.625 mg CE plus 2.5 mg MPA) significantly increased spine and total hip BMD by 4.5% and 3.7%, respectively, relative to placebo.
Effects of lower-than-standard doses of ET/EPT on BMD have been investigated. RCTs[247–251] using doses as low as 0.3 mg/day oral CE, 0.25 mg/day oral micronized 17β-estradiol, and 0.014 mg/day transdermal 17β-estradiol reported significant increases in spine and hip BMD relative to placebo. These trials were conducted either in populations of early postmenopausal women (mean age, 51–52 y) or in older postmenopausal women (mean ages, 67–74 y). Changes in lumbar spine BMD were in the range of 1% to 3%, significantly better than placebo.
Significant BMD improvements have also been noted with systemic estrogen doses delivered via a vaginal ring (Femring).[252] In an RCT of 174 postmenopausal women younger than age 65, daily doses of 0.05 and 0.1 mg of estradiol acetate delivered via the ring significantly increased hip BMD (1.7% and 1.8%, respectively) and lumbar spine BMD (2.7% and 3.3%) compared with baseline.
Fracture Evidence from both RCTs and observational studies indicate that standard doses of ET/EPT (including 0.625 mg CE/d or the equivalent) reduce fracture risk in postmenopausal women. Two meta-analyses have found that ET/EPT significantly reduces the risk of fracture by up to 27%.[253,254]
Two large observational studies support these data. The National Osteoporosis Risk Assessment (NORA) study examined 200,160 postmenopausal women and reported that current estrogen use was associated with a significantly reduced risk for new fracture.[86] Participants were at least age 50 and had had no previous diagnosis of osteoporosis. The Million Women Study,[255] a prospective observational study of 138,737 postmenopausal women, reported that ET/EPT use provided a significant RR reduction in incidence of fracture.
Results were confirmed in the WHI. In both the EPT arm[246] and the ET arm,[256] significant risk reductions were seen for hip fractures, vertebral fractures, and total fractures compared with placebo. The selection criteria and outcomes evaluated in the WHI (ie, women were not selected on the basis of an established osteoporosis risk factor or BMD level; fracture outcomes included hip, wrist/lower arm, and clinically identified vertebral and total fractures) are in contrast to the design of studies of fracture risk reduction with bisphosphonates or SERMs.[39,199,201,202,205,206] In those studies, women were selected on the basis of high risk for osteoporosis (ie, prevalent vertebral fracture and/or low BMD), and radiography-detected vertebral fractures were often a primary outcome.
The Million Women Study,[255] although observational in design, addressed issues related to ET/EPT and the risk of fracture that could not be ascertained in the WHI trials, such as comparisons between different EPT formulations, doses, and routes of administration. When the overall fracture-risk reduction was examined by type of hormone, no difference was found between ET and EPT. Sequential or continuous progestin use also did not significantly affect the results. Furthermore, the RR of fracture was not different when specific estrogen or progestogen products were compared (ie, CE versus estradiol; MPA versus norethisterone or norgestrel/levonorgestrel). This study did not specifically report on the possible fracture protection afforded by a low estrogen dose (ie, 0.3 mg), but found that risk reductions for doses greater than 0.625 mg were similar to those for doses 0.625 mg or less.
Therapy Management The primary indication for systemic ET/EPT is for women experiencing moderate to severe menopause symptoms (eg, vasomotor symptoms, vaginal atrophy).
In the WHI, systemic EPT (CE plus MPA) at standard doses for 5.6 years in postmenopausal women ages 50 to 79 was associated with a statistically significant increased risk of breast cancer,[257] stroke,[258,259] and thromboembolic events.[260] In women who had undergone a hysterectomy, ET alone for 6.8 years resulted in a statistically significant increased risk of stroke and deep venous thrombosis, whereas breast cancer, coronary heart disease, total VTE, and pulmonary embolism were not statistically increased.[256] For postmenopausal women ages 65 to 79 followed for a mean of 4.0 years, the Women's Health Initiative Memory Study[261] found a statistically significant increase in probable dementia for those who were receiving EPT. After a mean follow-up of 5.2 years, there was a nonsignificant trend for increased probable dementia among women allocated to ET alone.
NAMS recommends use of ET/EPT at the lowest effective dose consistent with treatment goals.[262] Lower doses of ET/EPT than used in the WHI, however, have not been examined with regard to fracture efficacy. Extended use of HT is an option for women who have established reduction in bone mass, regardless of menopause symptoms, for prevention of further bone loss and/or reduction of osteoporotic fracture when alternate therapies are not appropriate or cause side effects, or when the benefits of extended use are expected to exceed the risks. The optimal time to initiate ET/EPT and the optimal duration of therapy have not been established, but ET/EPT would largely be utilized in the early years after menopause. The benefits of HT on bone mass dissipate quickly after discontinuation of treatment.
Discontinuation of Therapy Studies have shown a BMD loss of 3% to 6% during the first year after cessation of systemic ET/EPT.[220,263–266] Data also indicate that the fracture risk reduction with ET/EPT does not persist after discontinuation of therapy. In the Million Women Study,[255] past users of hormone therapy had no protection against fracture, and incidence rates returned to those of never-users within about 1 year of ceasing use. In the NORA study,[267] clinical fractures of the hip, spine, forearm, wrist, or rib were reduced in current ET/EPT users but not in women who had stopped 5 years previously. In a further analysis of hip fractures, women who had discontinued ET/EPT within the previous 5 years had a risk for hip fracture at least as high as that in women who had never used ET/EPT.[268]
Calcitonin
Salmon calcitonin is government approved for postmenopausal osteoporosis treatment but not for prevention.[269] It is available in the United States as a nasal spray (marketed as Miacalcin Nasal Spray, Fortical Nasal Spray) and a subcutaneous injection (marketed as Miacalcin Injection). Available in Canada are a nasal spray (Miacalcin Nasal Spray and generics) and an injectable form (marketed as Calcimar Solution, Caltine), although these injectables are not indicated for osteoporosis.
Calcitonin is an inhibitor of bone resorption. In clinical use, however, the reduction in bone turnover with calcitonin is much less than with other antiresorptive agents. A small, dose-finding study of intranasal calcitonin in postmenopausal women with osteoporosis showed significant increases in spinal BMD of 3% relative to baseline.[270]
In the Prevent Recurrence of Osteoporotic Fractures (PROOF) trial,[271] an RCT, intranasal-spray calcitonin doses of 200 IU/day for 5 years significantly reduced the risk of new vertebral fracture by 33% compared with placebo in 1,255 postmenopausal women with established osteoporosis. No effect was seen for nonvertebral or hip fractures. However, statistically significant fracture reductions were not observed at either 100 IU/day or 400 IU/day. After 5 years, a significant spinal BMD increase compared with placebo was seen only for recipients of the 400-IU dose. No major effect on hip BMD occurred at any dose. The absence of a dose response as well as a 60% dropout rate have led some experts to doubt the reliability of these data.
Calcitonin has been shown to reduce bone pain from osteoporotic vertebral compression fractures more quickly than placebo immediately after a fracture;[272,273] however, it has not been shown to decrease bone pain in other situations.[274] Drug-related adverse effects include nausea, local inflammation, and flushing of the face or hands when calcitonin is given as an injection, and local nasal irritation with the nasal spray formulation.
Because calcitonin is a less effective agent than other pharmacologic therapies for osteoporosis, it is reserved as an alternative for women who cannot or choose not to take one of the other osteoporosis agents. The efficacy of calcitonin has not been observed in early postmenopausal women. Thus, product labeling recommends its use only in women with osteoporosis who are at least 5 years beyond menopause.
Combination Therapies
Combining potent antiresorptive agents results in small additional increments in bone density. In postmenopausal women (mean age, 61–62 y) with low bone mass, BMD improvements in the spine and hip with combined alendronate and ET were significantly greater (8.3%) than results for either agent alone (6.0%).[275] Combined risedronate and ET/EPT also has shown favorable, although modest, BMD effects compared with either agent alone.[276] Whether increases in BMD result in better fracture protection is not known, and the long-term safety of combination therapies has not been evaluated. One concern is that combining two antiresorptive therapies might oversuppress bone turnover, adversely affect bone quality, and thereby increase the likelihood of fracture. Combining antiresorptive agents is not generally recommended.
Combining an anabolic agent such as teriparatide with an antiresorptive agent has been considered. Significant increases in BMD occurred in an RCT when teriparatide was added to ongoing ET.[238] When PTH 1–84 and alendronate were combined, the BMD response was less than that seen with PTH alone.[241] Based on available data, recommendations cannot be made for or against combining antiresorptive and anabolic drugs.
Tibolone
Tibolone is approved in many countries, but not the United States or Canada, for the prevention of osteoporosis. In the Long-term Intervention on Fractures with Tibolone (LIFT) study,[277] tibolone reduced the risk of vertebral and nonvertebral fracture, breast cancer, and possibly colon cancer, but increased the risk of stroke in older postmenopausal women with osteoporosis.
Promising New Therapies
Several new drugs show promise for the treatment and/or prevention of osteoporosis. Some are now available outside North America, and others are in clinical development. These include strontium ranelate, PTH 1-84, additional SERMs (basedoxifene, lasofoxifene), oral calcitonin, denosumab, and odanacatib, an inhibitor of cathepsin K. This document will summarize data only for the therapies that have demonstrated fracture efficacy in published trials.
Strontium Ranelate
Oral strontium ranelate (marketed as Protelos) is approved for the prevention and treatment of osteoporosis in many countries outside North America. Dosing involves dissolving 2 grams of strontium ranelate in water and drinking it before bedtime. Other strontium salts are available as supplements, but no studies are available evaluating their effectiveness and safety.
A large RCT in postmenopausal women in Europe and Australia with the primary end point of vertebral fractures[278]demonstrated that 3 years of therapy significantly increased bone density at the spine (14%) and femoral neck (8%). Compared with placebo, the risk of spine fractures in strontium-treated women was significantly reduced by 49% after 1 year and 41% after 3 years compared with placebo. A second RCT investigating nonvertebral fractures[279] reported that after 3 years, nonvertebral fractures were significantly reduced by 16% in treated women compared with placebo. In a subgroup of high-risk women (older than age 74 with a femoral neck T-score of less than −2.4), there was a 36% decrease in hip fractures. Modest changes in markers of bone turnover have been observed with strontium therapy, but the exact mechanism by which strontium ranelate exerts its effect is unknown. Drug-related adverse effects included significant increases in nausea and diarrhea that resolved after 3 months, as well as VTE. During postmarketing surveillance, rare cases of hypersensitivity syndrome or DRESS (Drug Rash with Eosinophilia and Systemic Symptoms) were reported. Bone density in patients taking strontium ranelate will be artifactually increased by the effects of the higher atomic number of strontium ranelate as compared with calcium.
Parathyroid Hormone 1–84 The full-length PTH, PTH 1–84, is marketed in Europe and other countries as PreOs. In an RCT of 2,532 women with postmenopausal osteoporosis, PTH 1–84 administered as a daily subcutaneous injection in a 100-μg dose increased BMD in the lumbar spine by 6.9% and in the total hip region by 2.1% compared with placebo.[280] Vertebral fracture risk was reduced by 58%. No effect was observed on nonspine fractures. Hypercalcemia and hypercalciuria occurred more commonly with PTH 1–84 compared with placebo.
Bazedoxifene This SERM has prevented bone loss and decreased bone turnover without stimulating the endometrium in healthy postmenopausal women with normal or low BMD.[281] In a 3-year RCT involving 6,847 postmenopausal women with osteoporosis (average age, 66 y), bazedoxifene 20 or 40 mg/day reduced the incidence of vertebral fracture by 42% and 37%, respectively, in the active control group.[282] Overall, no effect was observed on nonvertebral fractures. The tolerability profile of bazedoxifene treatment was similar to that of raloxifene and included an increased incidence of vasomotor symptoms, VTE, and leg cramps compared with placebo.
Lasofoxifene Lasofoxifene is another SERM that increased lumbar spine BMD and reduced bone markers modestly more than did raloxifene in young postmenopausal women without osteoporosis.[283] In a phase 3 trial involving 8,556 postmenopausal women with osteoporosis, lasofoxifene in daily doses of 0.25 mg and 0.5 mg significantly reduced vertebral fracture risk compared with placebo by 31% and 42%, respectively.[284] The higher dose also significantly reduced the incidence of nonvertebral fractures by 22%. In that study, lasofoxifene significantly decreased the incidence of ER-positive breast cancer. The incidence of VTE was increased with both doses of therapy, similar to the effects seen with estrogen and other SERMs. No significant treatment effects were observed on the incidence of stroke or coronary heart disease.
Denosumab Denosumab is a fully human monoclonal antibody to receptor activator of nuclear factor-κB ligand (RANKL), a member of the tumor necrosis factor superfamily expressed on the surface of osteoblasts. RANKL binding to its receptor RANK on the surface of osteoclast precursors promotes the proliferation and differentiation of osteoclasts. By blocking the interaction between RANKL and RANK, denosumab inhibits bone resorption by osteoclasts. Denosumab is dosed as a subcutaneous injection every 6 months. In postmenopausal women with low bone mass, denosumab increased BMD in various skeletal sites similar to or slightly more than did alendronate given 70 mg/week.[285] In a phase 3 study of 7,808 women with osteoporosis, denosumab reduced the incidence of vertebral fractures by 68%, hip fracture by 40%, and nonspine fractures by 20% compared with placebo.[286]
BMD of the lumbar spine and total hip regions increased with denosumab therapy, compared with placebo, by 9.2% and 6.0%, respectively. The drug was well tolerated. Skin infections occurred more commonly with treatment than with placebo.
Recommendations
Management strategies for osteoporosis in postmenopausal women require assessment of risk factors for BMD-defined osteoporosis and osteoporotic fracture, followed by institution of measures that focus on reducing risk factors through lifestyle changes and, if indicated, pharmacologic therapy.
- All postmenopausal women should be encouraged to employ lifestyle practices that reduce the risk of bone loss and osteoporotic fractures: maintaining a healthy weight, eating a balanced diet, obtaining adequate calcium and vitamin D, participating in appropriate exercise, avoiding excessive alcohol consumption, not smoking, and utilizing measures to prevent falls. Periodic reviews of calcium and vitamin D intake and lifestyle behaviors are useful. After menopause, a woman's risk of falls should be assessed annually and at any time her physical or mental status changes.
- The physical examination should include an annual measurement of height and weight, along with an assessment for chronic back pain, kyphosis, and clinical risk factors.
- BMD testing is indicated for:
- All postmenopausal women with medical causes of bone loss
- All women age 65 and over
- BMD testing should be considered for postmenopausal women age 50 and older who have one or more of the following risk factors:
- Previous fracture (other than skull, facial bone, ankle, finger, and toe) after menopause
- Thinness (body weight < 127 lbs [57.7 kg] or BMI < 21 kg/m2)
- History of hip fracture in a parent
- Current smoking
- Rheumatoid arthritis
- Excessive alcohol intake
- When BMD testing is indicated, DXA is the preferred technique. The total hip, femoral neck, and posterior-anterior lumbar spine should be measured, using the lowest of the three BMD scores.
- The routine use of biochemical markers of bone turnover in clinical practice is not generally recommended.
- Vertebral fracture must be confirmed by lateral spine radiographs or VFA visualization of fracture at the time of BMD testing. Vertebral fracture is confirmed by height loss >20% of the anterior, mid, or posterior dimension of a vertebra on imaging.
- An adequate intake of both calcium and vitamin D is important for bone health and is recognized as an important component of any osteoporosis prescription-drug regimen. NAMS follows the NOF recommendations of calcium intake of 1,200 mg/day for adults age 50 and older, and vitamin D3 of 800 to 1,000 IU/day.
- NAMS recommends osteoporosis drug therapy in the following populations:
- All postmenopausal women who have had an osteoporotic vertebral or hip fracture
- All postmenopausal women who have BMD values consistent with osteoporosis (ie, T-scores ≤−2.5) at the lumbar spine, femoral neck, or total hip region
- All postmenopausal women who have T-scores from −1.0 to −2.5 and a 10-year risk, based on the FRAX calculator, of major osteoporotic fracture (spine, hip, shoulder, and wrist) of at least 20% or of hip fracture of at least 3%
- It is important to encourage adherence to the treatment plan and to identify barriers to nonadherence. Providing clear information to women regarding their risk for fracture and the purpose of osteoporosis therapy may be the optimal way to improve adherence.
- During therapy, it is appropriate to reevaluate the treatment goals and the choice of medication on an ongoing basis through periodic medical examination and a follow-up BMD testing. Measurement of BMD has limited use in predicting the effectiveness of antiresorptive therapies for reducing fracture risk. Also, fracture risk reductions from therapy occur much more rapidly than BMD changes. An appropriate interval for repeat BMD testing is after 1 to 2 years of treatment. There appears to be little value in repeat testing if a woman is stable (within the precision error of the original instrument).
- For untreated postmenopausal women, repeat DXA testing is not useful until 2 to 5 years have passed.
- Bisphosphonates are the first-line drugs for treating postmenopausal women with osteoporosis. They have reduced the risk of vertebral fractures by 40% to 70% and reduced the incidence of nonvertebral fracture, including hip fracture, by about half this amount.
- The SERM raloxifene is most often considered for postmenopausal women with low bone mass or younger postmenopausal women with osteoporosis. It prevents bone loss and reduces the risk of vertebral fractures, but its effectiveness in reducing other fractures is uncertain. Extraskeletal risks and benefits are important when considering raloxifene therapy.
- Teriparatide (PTH 1–34) is best offered to postmenopausal women with osteoporosis who are at high risk for fracture. Daily subcutaneous injections have been shown to stimulate bone formation and improve bone density. Therapy is indicated for no more than 24 months.
- The primary indication for systemic ET/EPT is to treat moderate to severe menopause symptoms (eg, vasomotor symptoms). When symptoms are controlled or cease, continued hormone therapy can still be considered for bone effects, weighing its benefits and risks against those of alternative therapies.
- ET/EPT may be a treatment option for a few years of early postmenopause.
- Calcitonin is not a first-line drug for postmenopausal osteoporosis treatment, as its fracture efficacy is not strong and its BMD effects are less than those of other agents. However, it is an option for women with osteoporosis who are more than 5 years beyond menopause. Calcitonin therapy may reduce vertebral fracture risk in women with osteoporosis, although the evidence documenting fracture protection is not strong. It is not recommended for treating bone pain, except bone pain from acute vertebral compression fractures.
- Data are inadequate to make definitive recommendations regarding combination or serial anabolic and antiresorptive drug therapies.
- The treatment of osteoporosis needs to be long term in most women.
- If drug-related adverse effects occur, appropriate management strategies should be instituted. If adverse effects persist, switching to another agent may be required.
- Decisions to discontinue or suspend therapy are based on the woman's risk of fracture and her response to treatment. Given the uncertainties of long-term drug safety, careful monitoring is required. Fracture risk after discontinuing therapy has not been adequately evaluated.
References
- The North American Menopause Society. The management of osteoporosis in postmenopausal women: 2006 position statement of The North American Menopause Society. Menopause 2006;13:340–367.
- Guyatt GH, Sackett DL, Sinclair JC, et al. Users' guides to the medical literature. IX. A method for grading health care recommendations. Evidence-Based Medicine Working Group. JAMA 1995;274:1800–1805.
- Jackson R, Feder G. Guidelines for clinical guidelines [editorial]. BMJ 1998;317:427–428.
- Scottish Intercollegiate Guidelines Network. SIGN guidelines: an introduction to SIGN methodology for the development of evidence-based clinical guidelines. Available at: http://www.sign.ac.uk/. Accessed August 22, 2009.
- Boggs P, Utian W. The North American Menopause Society develops consensus opinions [editorial]. Menopause1998;5:67–68.
- NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA 2001;85:785–795.
- Kanis JA. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group. Osteoporos Int 1994;4:368–381.
- Bonjour JP, Theintz G, Law F, Slosman D, Rizzoli R. Peak bone mass. Osteoporos Int 1994;4(suppl 1):S7–S13.
- Looker AC, Wahner HW, Dunn WL, et al. Updated data on proximal femur bone mineral levels of US adults.Osteoporos Int 1998;8:468–489.
- Eastell R. Forearm fracture. Bone 1996;18(suppl 3):S203–S207.
- Looker AC, Orwoll ES, Johnston CC Jr, et al. Prevalence of low femoral bone density in older U.S. adults from NHANES III. J Bone Miner Res 1997;12:1761–1768.
- Melton LJ III, Thamer M, Ray NF, et al. Fractures attributable to osteoporosis: report from the National Osteoporosis Foundation. J Bone Miner Res 1997;12:16–23.
- Siris ES, Chen YT, Abbott TA, et al. Bone mineral density thresholds for pharmacological intervention to prevent fractures. Arch Intern Med 2004;164:1108–1112.
- Stone KL, Seeley DG, Lui LY, et al, for the Study of Osteoporotic Fractures Research Group. BMD at multiple sites and risk of fracture of multiple types: long-term results from the Study of Osteoporotic Fractures. J Bone Miner Res2003;18:1947–1954.
- Wainwright SA, Marshall LM, Ensrud KE, et al, for the Study of Osteoporotic Fractures Research Group. Hip fracture in women without osteoporosis. J Clin Endocrinol Metab 2005;90:2787–2793.
- Johnell O, Kanis J. Epidemiology of osteoporotic fractures. Osteoporos Int 2005;16(suppl 2):S3–S7.
- Melton LJ III, Chrischilles EA, Cooper C, Lane AW, Riggs BL. How many women have osteoporosis? J Bone Miner Res 1992;7:1005–1010.
- Barrett-Connor E, Wehren LE, Siris ES, et al. Osteoporosis and fracture risk in women of different ethnic groups. J Bone Miner Res 2005;20:185–194.
- Cauley JA, Lui LY, Ensrud KE, et al. Bone mineral density and the risk of incident nonspinal fractures in black and white women. JAMA 2005;293:2102–2108.
- Leslie WD, O'Donnell S, Jean S, et al, for the Osteoporosis Surveillance Expert Working Group. Trends in hip fracture rates in Canada. JAMA 2009;302:883–889.
- Lindsay R, Silverman SL, Cooper C, et al. Risk of new vertebral fracture in the year following a fracture. JAMA2001;285:320–323.
- Klotzbuecher CM, Ros PD, Landsman PB, Abbott TA, Berger M. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res 2000;15:721–739.
- Silverman SL, Minshall ME, Shen W, Harper KD, Xie S, for the Health-Related Quality of Life Subgroup of the Multiple Outcomes of Raloxifene Evaluation Study. The relationship of health-related quality of life to prevalent and incident vertebral fractures in postmenopausal women with osteoporosis: results from the Multiple Outcomes of Raloxifene Evaluation Study. Arthritis Rheum 2001;44:2611–2619.
- Cauley JA, Thompson DE, Ensrud KC, Scott JC, Black D. Risk of mortality following clinical fractures. Osteoporos Int2000;11:556–561.
- Gold DT. The nonskeletal consequences of osteoporotic fractures: physiologic and social outcomes. Rheum Dis Clin North Am 2001;27:255–262.
- Riggs BL, Khosla S, Melton LJ III. A unitary model for involutional osteoporosis: estrogen deficiency causes both type I and type II osteoporosis in postmenopausal women and contributes to bone loss in aging men. J Bone Miner Res1998;13:763–773.
- Silverman SL. Selecting patients for osteoporosis therapy. Curr Osteoporos Rep 2006;4:91–95.
- World Health Organization Collaborating Centre for Metabolic Bone Diseases, University of Sheffield, UK FRAX®: WHO Fracture Risk Assessment Tool. Available at: http://www.shef.ac.uk/FRAX. Accessed August 19, 2009.
- Johnell O, Gullberg B, Kanis JA, et al. Risk factors for hip fracture in European women: the MEDOS Study. Mediterranean Osteoporosis Study. J Bone Miner Res 1995;10:1802–1815.
- Cummings SR, Black DM, Nevitt MC, et al. Bone density at various sites for prediction of hip fractures: the Study of Osteoporotic Fractures Research Group. Lancet 1993;341:72–75.
- Kanis JA, Glüer CC, for the Committee of Scientific Advisors, International Osteoporosis Foundation: an update on the diagnosis and assessment of osteoporosis with densitometry. Osteoporos Int 2000;11:192–202.
- Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ 1996;312:1254–1259.
- Kanis JA, for the World Health Organization Scientific Group. Assessment of Osteoporosis at the Primary Health Care Level. Technical Report. World Health Organization Collaborating Centre for Metabolic Bone Diseases. Sheffield, UK: University of Sheffield, 2008:100–131.
- Kanis JA, Johnell O, Oden A, Dawson A, De Laet C, Jonsson B. Ten-year probabilities of osteoporotic fractures according to BMD and diagnostic thresholds. Osteoporos Int 2001;12:989–995.
- Delmas PD, Seeman E. Changes in bone mineral density explain little of the reduction in vertebral or nonvertebral fracture risk with anti-resorptive therapy. Bone 2004;34:599–604.
- Hochberg MC, Ross PD, Black D, et al. Larger increases in bone mineral density during alendronate therapy are associated with a lower risk of new vertebral fractures in women with postmenopausal osteoporosis: Fracture Intervention Trial Research Group. Arthritis Rheum 1999;42:1246–1254.
- Watts NB, Cooper C, Lindsay R, et al. Relationship between changes in bone mineral density and vertebral fracture risk associated with risedronate: greater increases in bone mineral density do not relate to greater decreases in fracture risk. J Clin Densitom 2004;7:255–261.
- Roux C, Seeman E, Eastell R, et al. Efficacy of risedronate on clinical vertebral fractures within six months. Curr Med Res Opin 2004;20:433–439.
- Harris ST, Watts NB, Genant HK, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. JAMA 1999;282:1344–1352.
- Kanis JA, Johansson H, Oden A, et al. A family history of fracture and fracture risk: a meta-analysis. Bone2004;35:1029–1037.
- Black DM, Cummings SR, Karpt DB, et al. Randomized trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 1996;348:1535–1541.
- Cummings SR, Black DM, Thompson DE, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA 1998;280:2077–2082.
- Burger H, de Laet CE, van Daele, et al. Risk factors for increased bone loss in an elderly population: the Rotterdam study. Am J Epidemiol 1998;147:871–879.
- Slemenda CW, Christian JC, Williams CJ, et al. Genetic determinants of bone mass in adult women: a reevaluation of the twin model and the potential importance of gene interaction on heritability estimates. J Bone Miner Res1991;6:561–567.
- Pocock NA, Eisman JA, Hopper JL, et al. Genetic determinants of bone mass in adults: a twin study. J Clin Invest1987;80:706–710.
- Bauer DC, Browner WS, Cauley JA, et al. Factors associated with appendicular bone mass in older women: the Study of Osteoporotic Fractures Research Group. Ann Intern Med 1993;118:657–665.
- Seeman E, Hopper JL, Bach LA, et al. Reduced bone mass in daughters of women with osteoporosis. N Engl J Med1989;320:554–558.
- Evans RA, Marel GM, Lancaster EK, et al. Bone mass is low in relatives of osteoporotic patients. Ann Intern Med1988;109:870–873.
- Kanis JA, De Laet C, Delmas P, et al. A meta-analysis of previous fracture and fracture risk. Bone 2004;35:375–382.
- Cummings SR, Nevitt MC, Browner WS, et al. Risk factors for hip fracture in white women: Study of Osteoporotic Fractures Research Group. N Engl J Med 1995;332:767–773.
- van der Voort DJ, Geusens PP, Dinant GJ. Risk factors for osteoporosis related to their outcome: fractures.Osteoporos Int 2001;12:630–638.
- Recker RR, Lappe J, Davies K, Heaney R. Characterization of perimenopausal bone loss: a prospective study. J Bone Miner Res 2000;15:1965–1973.
- Pouilles JM, Tremollieres F, Ribot C. Vertebral bone loss in perimenopause: results of a 7-year longitudinal study.Presse Med 1996;25:277–280.
- Devine A, Dick IM, Dhaliwal SS, Naheed R, Beilby J, Prince RL. Prediction of incident osteoporotic fractures in elderly women using the free estradiol index. Osteoporos Int 2005;16:216–221.
- Pouilles JM, Tremollieres F, Bonneu M, Ribot C. Influence of early age at menopause on vertebral bone mass. J Bone Miner Res 1994;9:311–315.
- Ohta H, Sugimoto I, Masuda A, et al. Decreased bone mineral density associated with early menopause progresses for at least ten years: cross-sectional comparisons between early and normal menopausal women. Bone 1996;18:227–231.
- Gerdhem P, Obrant KJ. Bone mineral density in old age: the influence of age at menarche and menopause. J Bone Miner Metab 2004;22:372–375.
- Orstavik RE, Haugeberg G, Mowinckel P, et al. Vertebral deformities in rheumatoid arthritis: a comparison with population-based controls. Arch Intern Med 2004;164:420–425.
- Fujita K, Kasayama S, Hashimoto J, et al. Inhaled corticosteroids reduce bone mineral density in early postmenopausal but not premenopausal asthmatic women. J Bone Miner Res 2001;16:782–787.
- van Staa TP, Leufkens HG, Cooper C. Use of inhaled corticosteroids and risk of fractures. J Bone Miner Res2001;16:581–588.
- van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C. Oral corticosteroids and fracture risk: relationship to daily and cumulative doses. Rheumatology 2000;39:1383–1389.
- Sagsveen M, Farmer JE, Prentice A, Breeze A. Gonadotrophin-releasing hormone analogues for endometriosis: bone mineral density. Cochrane Database Syst Rev 2003;4:CD001297.
- Clark MK, Sowers MR, Nichols S, Levy B. Bone mineral density changes over two years in first-time users of depot medroxyprogesterone acetate. Fertil Steril 2004;82:1580–1586.
- Scholes D, LaCroix AZ, Ichikawa LE, Barlow WE, Ott SM. Injectable hormone contraception and bone density: results from a prospective study. Epidemiology 2002;13:581–587.
- Eastell R, Hannon RA, Cuzick J, Dowsett M, Clack G, Adams JE, for the ATAC Trialists' group. Effect of an aromatase inhibitor on BMD and bone turnover markers: 2-year results of the Anastrozole, Tamoxifen, Alone or in Combination (ATAC) trial. J Bone Miner Res 2006;21:1215–1223.
- Bell R, Lewis J. Assessing the risk of fracture in postmenopausal women who are receiving adjuvant hormonal therapy for breast cancer. Curr Med Res Opin 2007;23:1045–1051.
- Delmas PD, Eastell R, Garnero P, Seibel MJ, Stepan J, for the Committee of Scientific Advisors of the International Osteoporosis Foundation. The use of biochemical markers of bone turnover in osteoporosis. Committee of Scientific Advisors of the International Osteoporosis Foundation. Osteoporos Int 2000;11(suppl 6):S2–S17.
- Garnero P. Markers of bone turnover for the prediction of fracture risk. Osteoporosis Int 2000;11(Suppl 16):S55–S65.
- Berger C, Langsetmo L, Joseph L, et al, for the CaMOS Research Group. Association between change in bone mineral density (BMD) and fragility fracture in women and men. J Bone Miner Res 2009;24:361–370.
- van Staa TP, Laan RF, Barton IP, Cohen S, Reid DM, Cooper C. Bone density threshold and other predictors of vertebral fracture in patients receiving oral glucocorticoid therapy. Arthritis Rheum 2003;48:3224–3229.
- Siminoski K, Jiang G, Adachi JD, et al. Accuracy of height loss during prospective monitoring for detection of incident vertebral fractures. Osteoporos Int 2005;16:403–410.
- Wasnich RD. Vertebral fracture epidemiology. Bone 1996;18(suppl):179S-183S.
- Wu CY, Li J, Jergas M, Genant HK. Comparison of semiquantitative and quantitative techniques for the assessment of prevalent and incident vertebral fractures. Osteoporos Int 1995;5:354–370.
- Hedlund LR, Gallagher JC, Meeger C, Stoner S. Change in vertebral shape in spinal osteoporosis. Calcif Tissue Int1989;44:168–172.
- Majumdar SR, Kim N, Colman I, et al. Incidental vertebral fractures discovered with chest radiography in the emergency department: prevalence, recognition, and osteoporosis management in a cohort of elderly patients. Arch Intern Med 2005;165:905–909.
- Schneider DL, von Muhlen D, Barrett-Connor E, Sartoris DJ. Kyphosis does not equal vertebral fractures: the Rancho Bernardo study. J Rheumatol 2004;31:747–752.
- Greenspan SL, von Stetten E, Emond SK, Jones L, Parker RA. Instant vertebral assessment: a noninvasive dual x-ray absorptiometry technique to avoid misclassification and clinical mismanagement of osteoporosis. J Clin Densitom2001;4:373–380.
- Ferrar L, Jiang G, Barrington NA, Eastell R. Identification of vertebral deformities in women: comparison of radiological assessment and quantitative morphometry using morphometric radiography and morphometric x-ray absorptiometry. J Bone Miner Res 2000;15:575–585.
- Genant HK, Wu CY, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 1993;8:1137–1148.
- Genant HK, Jergas M, Palermo L, et al. Comparison of semiquantitative visual and quantitative morphometric assessment of prevalent and incident vertebral fractures in osteoporosis: the Study of Osteoporotic Fractures Research Group. J Bone Miner Res 1996;11:984–996.
- Leipzig RM, Cumming RG, Tinetti ME. Drugs and falls in older people: a systematic review and meta-analysis. J Am Geriatr Soc 1999;47:30–50.
- Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med 1988;319:1701–1707.
- Iwamoto J, Suzuki H, Tanaka K, et al. Preventative effect of exercise against falls in the elderly: a randomized controlled trial. Osteoporos Int 2009;20:1233–1240.
- Fortinsky RH, Baker D, Gottschalk M, King M, Trella P, Tinetti ME. Extent of implementation of evidence-based fall prevention practices for older patients in home health care. J Am Geriatr Soc 2008;56:737–743.
- Kanis JA, Borgstrom F, De Laet C, et al. Assessment of fracture risk. Osteoporos Int 2005;16:581–589.
- Siris ES, Miller PD, Barrett-Connor E, et al. Identification and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women: results from the National Osteoporosis Risk Assessment. JAMA 2001;286:2815–2822.
- Hodgson SF, Watts NB, Bilezikian JP, et al, for the American Association of Clinical Endocrinologists. American Association of Clinical Endocrinologists 2001 medical guidelines for clinical practice for the prevention and management of postmenopausal osteoporosis. Endocr Pract 2001;7:293–312.
- Knoke JD, Barrett-Connor E. Weight loss: a determinant of hip bone loss in older men and women. The Rancho Bernardo Study. Am J Epidemiol 2003;158:1132–1138.
- Schousboe JT, Bauer DC, Nyman JA, Kane RL, Melton LJ, Ensrud KE. Potential for bone turnover markers to cost-effectively identify and select post-menopausal osteopenic women at high risk of fracture for bisphosphonate therapy.Osteoporos Int 2007;18:201–210.
- Delmas P, Munoz F, Black D, et al, for the HORIZON-PFT Research Group. Effects of yearly zoledronic acid 5 mg on bone turnover markers and relation of PINP with fracture reduction in postmenopausal women with osteoporosis. J Bone Miner Res 2009;25:1544–1551.
- Marcus R, Holloway L, Wells B, et al. The relationship of biochemical markers of bone turnover to bone density changes in postmenopausal women: results from the Postmenopausal Estrogen/Progestin Interventions (PEPI) trial. J Bone Miner Res 1999;14:1583–1595.
- Miller PD, Baran DT, Bilezikian JT, et al. Practical clinical applications of biochemical markers of bone turnover: consensus of an expert panel. J Clin Densitom 1999;2:323–342.
- Miller PD, Hochberg MC, Wehren LE, Ross PD, Wasnich RD. How useful are measures of BMD and bone turnover?Curr Med Res Opin 2005;21:545–554.
- Bell KJ, Hayen A, Macaskill P, et al. Value of routine monitoring of bone mineral density after starting bisphosphonate treatment: secondary analysis of trial data. BMJ 2009;338:b2266.
- Ensrud KE, Ewing SK, Stone KL, Cauley JA, Bowman PJ, Cummings SR, for the Study of Osteoporotic Fractures Research Group. Intentional and unintentional weight loss increase bone loss and hip fracture risk in older women. J Am Geriatr Soc 2003;51:1740–1747.
- Ervin RB. Healthy eating index scores among adults, 60 years of age and over, by sociodemographic and health characteristics: United States, 1999–2002. Adv Data 2008;395:1–16.
- Prince RL, Devine A, Dhaliwal SS, Dick IM. Effects of calcium supplementation on clinical fracture and bone structure: results of a 5-year, double-blind, placebo-controlled trial in elderly women. Arch Intern Med 2006;166:869–875.
- Jackson RD, LaCroix AZ, Gass M, et al, for the Women's Health Initiative Investigators. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med 2006;354:669–683.
- Ervin RB, Wang CY, Wright JD, Kennedy-Stephenson J. Dietary intake of selected minerals for the United States population: 1999–2000. Adv Data 2004;341:1–5.
- Ireland P, Fordtran JS. Effect of dietary calcium and age on jejunal calcium absorption in humans studies by intestinal perfusion. J Clin Invest 1973;52:2672–2681.
- Holick MF, Siris ES, Binkley N, et al. Prevalence of vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab 2005;90:3215–3224.
- Lips P, Duong T, Oleksik A, et al. A global study of vitamin D status and parathyroid function in postmenopausal women with osteoporosis: baseline data from the multiple outcomes of raloxifene evaluation clinical trial. J Clin Endocrinol Metab 2001;86:1212–1221.
- Heaney RP, Recker RR, Ryan RA. Urinary calcium in perimenopausal women: normative values. Osteoporos Int1999;9:13–18.
- National Osteoporosis Foundation. Clinician's Guide to Prevention and Treatment of Osteoporosis. Washington, DC: National Osteoporosis Foundation, 2008. Available at: http://www.nof.org/professionals/NOF_Clinicians_Guide.pdf. Accessed August 22, 2009.
- National Institutes of Health. NIH consensus development panel on optimal calcium Intake. Optimal calcium intake.JAMA 1994;272:1942–1948.
- Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Food and Nutrition Board Institute of Medicine. Dietary Reference Intakes: Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. Washington, DC: National Academy Press, 1997.
- Brown JP, Josse RG. 2002 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada.CMAJ 2002;167(Suppl 10):S1–34.
- Statistics Canada. Canadian community health survey, cycle 2.2, nutrition (2004). Nutrient intakes from food. Provincial, regional and national summary data tables, vol 1. http://www.hc-sc.gc.ca/fn-an/surveill/nutrition/commun/index-eng.php. Accessed September 3, 2009.
- Wang L, Manson JE, Buring JE, Lee IM, Sesso HD. Dietary intakes of dairy products, calcium, and vitamin D and the risk of hypertension in middle-aged and older women. Hypertension 2008;51:1073–1079.
- The North American Menopause Society. The role of calcium in peri- and postmenopausal women: 2006 position statement of The North American Menopause Society. Menopause 2006;13:862–877.
- Heaney RP. Calcium supplementation and incident kidney stone risk: a systematic review. J Am Coll Nutr2008;27:519–527.
- Heaney RP. Optimal vitamin D status. J Bone Miner Res 2009;24:755.
- Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status.Osteoporos Int 2005;16:713–716.
- Bischoff-Ferrari HA, Giovannucci E, Willett WC, et al. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr 2006;84:18–28.
- Holick MF. Vitamin D: the underappreciated D-lightful hormone that is important for skeletal and cellular health. Curr Opin Endocrinol Diabetes 2002;9:87–98.
- American Academy of Dermatology and AAD Association. Position statement on vitamin D. Available at: http://www.aad.org/Forms/Policies/Uploads/PS/PS-Vitamin%20D.pdf. Accessed September 3, 2009.
- Binkley N, Novotny R, Krueger D, et al. Low vitamin D status despite abundant sun exposure. J Clin Endocrinol Metab2007;92:2130–2135.
- Hathcock JN, Shao A, Vieth R, Heaney R. Risk assessment for vitamin D. Am J Clin Nutr 2007;85:6–18.
- Trang HM, Cole DE, Rubin LA, et al. Evidence that vitamin D3 increases serum 25-hydroxyvitamin D more efficiently than does vitamin D2. Am J Clin Nutr 1998;68:854–858.
- Armas LAG, Hollis BW, Heaney RP. Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab 2004;89:5387–5391.
- Holick MF, Biancuzzo RM, Chen TC, et al. Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D. J Clin Endocrinol Metab 2008;93:677–681.
- Heaney RP. Functional indices of vitamin D status and ramifications of vitamin D deficiency. Am J Clin Nutr2004;80(suppl):1706S-1709S.
- Grant AM, Avenell A, Campbell MK, et al, for the RECORD Trial Group. Oral vitamin D3 and calcium for secondary prevention of low-trauma fractures in elderly people (Randomised Evaluation of Calcium Or vitamin D, RECORD): a randomised placebo-controlled trial. Lancet 2005;365:1621–1628.
- Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B. Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. JAMA 2005;293:2257–2264.
- Dawson-Hughes B, Dallal GE, Krall EA, et al. A controlled trial of the effect of calcium supplementation on bone density in postmenopausal women. N Engl J Med 1990;23:878–883.
- Bischoff HA, Stahelin HB, Dick W, et al. Effects of vitamin D and calcium supplementation on falls: a randomized controlled trial. J Bone Miner Res 2003;18:343–351.
- Pfeifer M, Begerow B, Minne HW, Abrams C, Nachtigall D, Hansen C. Effects of a short-term vitamin D and calcium supplementation on body sway and secondary hyperparathyroidism in elderly women. J Bone Miner Res2000;15:1113–1118.
- Bischoff-Ferrari HA, Conzelmann M, Stähelin HB, et al. Is fall prevention by vitamin D mediated by a change in postural or dynamic balance? Osteoporos Int 2006;17:656–663.
- Bischoff-Ferrari HA, Dawson-Hughes B, Willett WC, et al. Effect of vitamin D on falls: a meta-analysis. JAMA2004;291:1999–2006.
- Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington, DC: National Academy Press, 2001.
- Braam LA, Knapen MH, Geusens P, et al. Vitamin K1 supplementation retards bone loss in postmenopausal women between 50 and 60 years of age. Calcif Tissue Int 2003;73:21–26.
- Bolton-Smith C, McMurdo ME, Paterson CR, et al. Two-year randomized controlled trial of vitamin K1 (phylloquinone) and Vitamin D3 plus calcium on the bone health of older women. J Bone Miner Res 2007;22:509–519.
- Cheung AM, Tile L, Lee Y, et al. Vitamin K supplementation in postmenopausal women with osteopenia (ECKO Trial): a randomized controlled trial. PLoS Med 2008;5:e196.
- Mutlu M, Argun M, Kilic E, Saraymen R, Yazar S. Magnesium, zinc and copper status in osteoporotic, osteopenic and normal post-menopausal women. J Int Med Res 2007;35:692–695.
- Nieves JW. Osteoporosis: the role of micronutrients. Am J Clin Nutr 2005;81:1232S-1239S.
- Odabasi E, Turan M, Aydin A, Akay C, Kutlu M. Magnesium, zinc, copper, manganese, and selenium levels in postmenopausal women with osteoporosis. Can magnesium play a key role in osteoporosis? Ann Acad Med Singapore2008;37:564–567.
- Spencer H, Fuller H, Norris C, Williams D. Effect of magnesium on the intestinal absorption of calcium in man. J Am Coll Nutr 1994;13:485–492.
- Durlach J, Bac P, Durlach V, Rayssiguier Y, Bara M, Guiet-Bara A. Magnesium status and ageing: an update. Magnes Res 1998;11:25–42.
- Rude RK, Olerich M. Magnesium deficiency: possible role in osteoporosis associated with gluten-sensitive enteropathy. Osteoporos Int 1996;6:453–461.
- Hannan MT, Tucker KL, Dawson-Hughes B, Cupples LA, Felson DT, Kiel DP. Effect of dietary protein on bone loss in elderly men and women: the Framingham Osteoporosis Study. J Bone Miner Res 2000;15:2504–2512.
- Rapuri PB, Gallagher JC, Haynatzka V. Protein intake: effects on bone mineral density and the rate of bone loss in elderly women. Am J Clin Nutr 2003;77:1517–1525.
- Tkatch L, Rapin CH, Rizzoli R, et al. Benefits of oral protein supplementation in elderly patients with fracture of the proximal femur. J Am Coll Nutr 1992;11:519–525.
- Heaney RP, Layman DK. Amount and type of protein influences bone health. Am J Clin Nutr 2008;87:1567S-1570S.
- Branca F. Dietary phyto-oestrogens and bone health. Proc Nutr Soc 2003;62:877–887.
- Alexandersen P, Toussaint A, Christiansen C, et al. Ipriflavone in the treatment of postmenopausal osteoporosis: a randomized controlled trial. Ipriflavone Multicenter European Fracture Study. JAMA 2001;285:1482–1488.
- Chen YM, Ho SC, Lam SS, Ho SS, Woo JL. Beneficial effect of soy isoflavones on bone mineral content was modified by years since menopause, body weight, and calcium intake: a double-blind, randomized, controlled trial. Menopause2004;11:246–254.
- Cassidy A, Albertazzi P, Lise Nielsen I, et al. Critical review of health effects of soyabean phyto-oestrogens in post-menopausal women. Proc Nutr Soc 2006;65:76–92.
- Ma DF, Qin LQ, Wang PY, Katoh R. Soy isoflavone intake increases bone mineral density in the spine of menopausal women: meta-analysis of randomized controlled trials. Clin Nutr 2008;27:57–64.
- Marini H, Minutoli L, Polito F, et al. Effects of the phytoestrogen genistein on bone metabolism in osteopenic postmenopausal women: a randomized trial. Ann Intern Med 2007;146:839–847.
- Kreijkamp-Kaspers S, Kok L, Grobbee DE, et al. Effect of soy protein containing isoflavones on cognitive function, bone mineral density, and plasma lipids in postmenopausal women: a randomized controlled trial. JAMA 2004;292:65–74.
- Gallagher JC, Satpathy R, Rafferty K, Haynatzka V. The effect of soy protein isolate on bone metabolism. Menopause2004;11:290–298.
- Atkinson C, Compston JE, Day NE, Dowsett M, Bingham SA. The effects of phytoestrogen isoflavones on bone density in women: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr 2004;79:326–333.
- Brink E, Coxam V, Robins S, et al. Long-term consumption of isoflavone-enriched foods does not affect bone mineral density, bone metabolism, or hormonal status in early postmenopausal women: a randomized, double-blind, placebo controlled study. Am J Clin Nutr 2008;87:761–770.
- Liu J, Ho SC, Su YX, et al. Effect of long-term intervention of soy isoflavones on bone mineral density in women: a meta-analysis of randomized controlled trials. Bone 2009;44:948–953.
- Lunt M, Masaryk P, Scheidt-Nave C, et al. The effects of lifestyle, dietary dairy intake and diabetes on bone density and vertebral deformity prevalence: the EVOS study. Osteoporos Int 2001;12:688–698.
- Wilsgaard T, Emaus N, Ahmed LA, et al. Lifestyle impact on lifetime bone loss in women and men: the Tromso Study.Am J Epidemiol 2009;169:877–886.
- Dook JE, James C, Henderson NK, Price RI. Exercise and bone mineral density in mature female athletes. Med Sci Sports Exerc 1997;29:291–296.
- Kannus P, Haapasalo H, Sievänen H, Oja P, Vuori I. The site-specific effects of long-term unilateral activity on bone mineral density and content. Bone 1994;15:279–284.
- Verschueren SM, Roelants M, Delecluse C, Swinnen S, Vanderschueren D, Boonen S. Effect of 6-month whole body vibration training on hip density, muscle strength, and postural control in postmenopausal women: a randomized controlled pilot study. J Bone Miner Res 2004;19:352–359.
- Rubin C, Recker R, Cullen D, Ryaby J, McCabe J, McLeod K. Prevention of postmenopausal bone loss by a low-magnitude, high-frequency mechanical stimuli: a clinical trial assessing compliance, efficacy, and safety. J Bone Miner Res 2004;19:343–351.
- Snow-Harter C, Bouxsein ML, Lewis BT, Carter DR, Marcus R. Effects of resistance and endurance exercise on bone mineral status of young women: a randomized exercise intervention trial. J Bone Miner Res 1992;7:761–769.
- Kelley GA, Kelley KS, Tran ZV. Exercise and lumbar spine bone mineral density in postmenopausal women: a meta-analysis of individual patient data. J Gerontol A Biol Sci Med Sci 2002;57:599–604.
- Notelovitz M, Martin D, Tesar R, et al. Estrogen therapy and variable-resistance weight training increase bone mineral in surgically menopausal women. J Bone Miner Res 1991;6:583–590.
- Robertson MC, Campbell AJ, Gardner MM, Devlin N. Preventing injuries in older people by preventing falls: a meta-analysis of individual-level data. J Am Geriatr Soc 2002;50:905–911.
- Sinkai M, Itoli E, Wahner HW, et al. Stronger back muscles reduce the incidence of vertebral fractures: a prospective 10 year follow-up of postmenopausal women. Bone 2002;30:836–841.
- Huntoon EA, Schmidt CK, Sinaki M. Significantly fewer refractures after vertebroplasty in patients who engage in back-extensor-strengthening exercises. Mayo Clin Proc 2008;83:54–57.
- Hongo M, Itoi E, Sinaki M, et al. Effect of low-intensity back exercise on quality of life and back extensor strength in patients with osteoporosis: a randomized controlled trial. Osteoporos Int 2007;18:1389–1395.
- Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet 2002;359:1761–1767.
- O'Loughlin JL, Robitaille Y, Boivin JF, Suissa S. Incidence of and risk factors for falls and injurious falls among the community-dwelling elderly. Am J Epidemiol 1993;137:342–354.
- Gillespie LD, Gillespie WJ, Robertson MC, Lamb SE, Cumming RG, Rowe BH. Interventions for preventing falls in elderly people. Cochrane Database Syst Rev 2003;4:CD000340.
- Campbell AJ, Robertson MC, Gardner MM, Norton RN, Buchner DM. Psychotropic medication withdrawal and a home-based exercise program to prevent falls: a randomized, controlled trial. J Am Geriatr Soc 1999;47:850–853.
- Cameron ID, Cumming RG, Kurrle SE, et al. A randomised trial of hip protector use by frail older women living in their own homes. Inj Prev 2003;9:138–141.
- Parker MJ, Gillespie WJ, Gillespie LD. Hip protectors for preventing hip fractures in older people. Cochrane Database Syst Rev 2005;3:CD001255.
- Slemenda CW, Hui SL, Longcope C, et al. Cigarette smoking, obesity, and bone mass. J Bone Miner Res 1989;4:737–741.
- Kato I, Toniolo P, Akhmedkhanov A, et al. Prospective study of factors influencing the onset of natural menopause. J Clin Epidemiol 1998;51:1271–1276.
- Krall EA, Dawson-Hughes B. Smoking and bone loss among postmenopausal women. J Bone Miner Res 1991;6:331–338.
- Baron JA, Farahmand BY, Weiderpass E, et al. Cigarette smoking, alcohol consumption, and risk for hip fracture in women. Arch Intern Med 2001;161:983–988.
- Kanis JA, Johnell O, Oden A, et al. Smoking and fracture risk: a meta-analysis. Osteoporos Int 2005;16:155–162.
- Rapuri PB, Gallagher JC, Balhorn KE, Ryschon KL. Smoking and bone metabolism in elderly women. Bone2000;27:429–436.
- Krall EA, Dawson-Hughes B. Smoking increases bone loss and decreases intestinal calcium absorption. J Bone Miner Res 1999;14:215–220.
- Jensen J, Christiansen C, Rodbro P. Cigarette smoking, serum estrogens and bone loss during hormone replacement therapy early after menopause. N Engl J Med 1985;313:973–975.
- Law MR, Hackshaw AK. A meta-analysis of cigarette smoking, bone mineral density and risk of hip fracture: recognition of a major effect. BMJ 1997;315:841–846.
- Tucker KL, Jugdaohsingh R, Powell JJ, et al. Effects of beer, wine, and liquor intakes on bone mineral density in older men and women. Am J Clin Nutr 2009;89:1188–1196.
- Felson DT, Zhang Y, Hannan MT, Kannel WB, Kiel DP. Alcohol intake and bone mineral density in elderly men and women: the Framingham Study. Am J Epidemiol 1995;142:485–492.
- Felson DT, Kiel DP, Anderson JJ, Kannel WB. Alcohol consumption and hip fractures: the Framingham Study. Am J Epidemiol 1988;128:1102–1110.
- Kool B, Ameratunga S, Robinson E, Crengle S, Jackson R. The contribution of alcohol to falls at home among working-aged adults. Alcohol 2008;42:383–388.
- Kanis JA, Johansson H, Johnell O, et al. Alcohol intake as a risk factor for fracture. Osteoporos Int 2005;16:737–742.
- McCombs JS, Thiebaud P, McLaughlin-Miley C, Shi J. Compliance with drug therapies for the treatment and prevention of osteoporosis. Maturitas 2004;48:271–287.
- Tosteson ANA, Grove MR, Hammond CS, et al. Early discontinuation of treatment for osteoporosis. Am J Med2003;115:209–216.
- Segal E, Tamir A, Ish-Shalom S. Compliance of osteoporotic patients with different treatment regimens. Isr Med Assoc J 2003;5:859–862.
- McClung M. Bisphosphonates. Endocrinol Metab Clin North Am 2003;32:253–271.
- Schnitzer T, Bone HG, Crepaldi G, et al. Therapeutic equivalence of alendronate 70 mg once-weekly and alendronate 10 mg daily in the treatment of osteoporosis. Alendronate Once-Weekly Study Group. Aging (Milano) 2000;12:1–12.
- Brown JP, Kendler DL, McClung MR, et al. The efficacy and tolerability of risedronate once a week for the treatment of postmenopausal osteoporosis. Calcif Tissue Int 2002;71:103–111.
- Miller PD, McClung MR, Macovei L, et al. Monthly oral ibandronate therapy in postmenopausal osteoporosis: 1-year results from the MOBILE study. J Bone Miner Res 2005;20:1315–1322.
- Boniva [package insert]. Roche Laboratories; 2008.
- McClung M, Clemmesen B, Daifotis A, et al. Alendronate prevents postmenopausal bone loss in women without osteoporosis: a double-blind, randomized, controlled trial. Ann Intern Med 1998;128:253–261.
- McClung MR, Wasnich RD, Hosking DJ, et al, for the Early Postmenopausal Intervention Cohort Study. Prevention of postmenopausal bone loss: six-year results from the Early Postmenopausal Intervention Cohort Study. J Clin Endocrinol Metab 2004;89:4879–4885.
- Liberman UA, Weiss SR, Broll JL. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. N Engl J Med 1995;333:1437–1443.
- Ensrud KE, Barrett-Connor EL, Schwartz A, et al, for the Fracture Intervention Trial Long-Term Extension Research Group. Randomized trial of effect of alendronate continuation versus discontinuation in women with low BMD: results from the Fracture Intervention Trial long-term extension. J Bone Miner Res 2004;19:1259–1269.
- Bone HG, Hosking D, Devogelaer JP, et al, for the Alendronate Phase III Osteoporosis Treatment Study Group. Ten years' experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med 2004;350:1189–1199.
- Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures: Fracture Intervention Trial Research Group. Lancet 1996;348:1531–1541.
- Black DM, Thompson DE, Bauer DC, et al. Fracture risk reduction with alendronate in women with osteoporosis: the Fracture Intervention Trial. Fracture Intervention Trial Research Group. J Clin Endocrinol Metab 2000;85:4118–4124.
- Mortensen L, Charles P, Bekker PJ, et al. Risedronate increases bone mass in an early postmenopausal population: two years of treatment plus one year of follow-up. J Clin Endocrinol Metab 1998;83:396–402.
- Mellstrom DD, Sorensen OH, Goemaere S, Roux C, Johnson TD, Chines AA. Seven years of treatment with risedronate in women with postmenopausal osteoporosis. Calcif Tissue Int 2004;75:462–468.
- Reginster J, Minne HW, Sorensen OH, et al. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int 2000;11:83–91.
- McClung MR, Geusens P, Miller PD, et al. Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med 2001;344:333–340.
- Sorensen OH, Crawford GM, Mulder H, et al. Long-term efficacy of risedronate: a 5-year placebo-controlled clinical experience. Bone 2003;32:120–126.
- McClung MR, Wasnich RD, Recker R, et al, for the Oral Ibandronate Study Group. Oral daily ibandronate prevents bone loss in early postmenopausal women without osteoporosis. J Bone Miner Res 2004;19:11–18.
- Chesnut CH III, Skag A, Christiansen C, et al. Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res 2004;19:1241–1249.
- Black DM, Delmas PD, Eastell R, et al, for the HORIZON Pivotal Fracture Trial. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 2007;356:1809–1822.
- Cranney A, Guyatt G, Krolicki N, et al. A meta-analysis of etidronate for the treatment of postmenopausal osteoporosis. Osteoporosis Research Advisory Group. Osteoporos Int 2001;12:140–151.
- Hodsman A, Adachi J, Olszynski W. Use of bisphosphonates in the treatment of osteoporosis: prevention and management of osteoporosis: consensus statements from the scientific advisory board of the Osteoporosis Society of Canada. Can Med Assoc J 1996;155(suppl):S945–S948.
- Odvina CV, Zerwekh JE, Rao DS, Maalouf N, Gottschalk FA, Pak CY. Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab 2005;90:1294–1304.
- Lenart BA, Neviaser AS, Lyman S, et al. Association of low-energy femoral fractures with prolonged bisphosphonate use: a case control study. Osteoporos Int 2008;20:1353–1362.
- Neviaser AS, Lane JM, Lenart BA, Edobor-Osula F, Lorich DG. Low-energy femoral shaft fractures associated with alendronate use. J Orthop Trauma 2008;22:346–350.
- Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff SL. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg 2004;62:527–534.
- Migliorati CA, Casiglia J, Epstein J, Jacobsen PL, Siegel MA, Woo S-B. Managing the care of patients with bisphosphonate-associated osteonecrosis: an American Academy of Oral Medicine position paper. J Am Dent Assoc 2005;136:1658–1668.
- Marx RE, Sawatari Y, Fortin M, Broumand V. Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition, prevention, and treatment. J Oral Maxillofac Surg 2005;63:1567–1575.
- Migliorati CA, Schubert MM, Peterson DE, Seneda LM. Bisphosphonate-associated osteonecrosis of mandibular and maxillary bone: an emerging oral complication of supportive cancer therapy. Cancer 2005;104:83–93.
- Wasnich RD, Bagger YZ, Hosking DJ, et al, for the Early Postmenopausal Intervention Cohort Study Group. Changes in bone density and turnover after alendronate or estrogen withdrawal. Menopause 2004;11:622–630.
- Curtis JR, Westfall AO, Cheng H, Delzell E, Saag KG. Risk of hip fracture after bisphosphonate discontinuation: implications for a drug holiday. Osteoporos Int 2008;19:1613–1620.
- Watts NB, Chines A, Olszynski WP, et al. Fracture risk remains reduced one year after discontinuation of risedronate.Osteoporos Int 2008;19:365–372.
- Delmas PD, Bjarnason NH, Mitlak BH, et al. Effects of raloxifene on bone mineral density, serum cholesterol concentrations, and uterine endometrium in postmenopausal women. N Engl J Med 1997;337:1641–1647.
- Ettinger B, Black DM, Mitlack BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA 1999;282:637–645.
- Delmas PD, Ensrud KE, Adachi JD, et al, for the Multiple Outcomes of Raloxifene Evaluation Investigators. Efficacy of raloxifene on vertebral fracture risk reduction in postmenopausal women with osteoporosis: four-year results from a randomized clinical trial. J Clin Endocrinol Metab 2002;87:3609–3617.
- Maricic M, Adachi JD, Sarkar S, Wu W, Wong M, Harper KD. Early effects of raloxifene on clinical vertebral fractures at 12 months in postmenopausal women with osteoporosis. Arch Intern Med 2002;162:1140–1143.
- Cummings SR, Eckert S, Krueger KA, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA 1999;281:2189–2197.
- Cauley JA, Norton L, Lippman ME, et al. Continued breast cancer risk reduction in postmenopausal women treated with raloxifene: 4-year results from the MORE trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators.Breast Cancer Res Treat 2001;65:125–134.
- Martino S, Cauley JA, Barrett-Connor E, et al, for CORE Investigators. Continuing outcomes relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst2004;96:1751–1761.
- Grady D, Ettinger B, Moscarelli E, et al, for the Multiple Outcomes of Raloxifene Evaluation Investigators. Safety and adverse effects associated with raloxifene: multiple outcomes of raloxifene evaluation. Obstet Gynecol 2004;104:837–844.
- Barrett-Connor E, Grady D, Sashegvi A, et al, for the MORE Investigators (Multiple Outcomes of Raloxifene Evaluation). Raloxifene and cardiovascular events in osteoporotic postmenopausal women: four-year results from the MORE (Multiple Outcomes of Raloxifene Evaluation) randomized trial. JAMA 2002;287:847–857.
- Ensrud K, Genazzani AR, Geiger MJ, et al. Effect of raloxifene on cardiovascular adverse events in postmenopausal women with osteoporosis. Am J Cardiol 2006;97:520–527.
- Barrett-Connor E, Mosca L, Collins P, et al, for the Raloxifene Use for The Heart Trial Investigators. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med 2006;355:125–137.
- Barrett-Connor E, Cox DA, Song J, Mitlak B, Mosca L, Grady D. Raloxifene risk for stroke based on the Framingham stroke risk score. Am J Med 2009;122:754–761.
- Neele SJ, Evertz R, De Valk-De Roo G, Roos JC, Netelenbos JC. Effect of 1 year of discontinuation of raloxifene or estrogen therapy on bone mineral density after 5 years of treatment in healthy postmenopausal women. Bone2002;30:599–603.
- Siris ES, Harris ST, Eastell R, et al, for the Continuing Outcomes Relevant to Evista (CORE) Investigators. Skeletal effects of raloxifene after 8 years: results from the continuing outcomes relevant to Evista (CORE) study. J Bone Miner Res 2005;20:1514–1524.
- Dempster DW, Cosman F, Kurland ES, et al. Effects of daily treatment with parathyroid hormone on bone microarchitecture and turnover in patients with osteoporosis: a paired biopsy study. J Bone Miner Res 2001;16:1846–1853.
- Lindsay R, Nieves J, Formica C, et al. Randomised controlled study of effect of parathyroid hormone on vertebral-bone mass and fracture incidence among postmenopausal women on oestrogen with osteoporosis. Lancet 1997;350:550–555.
- Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 2001;344:1434–1441.
- Forteo [package insert]. Eli Lilly and Company; 2004.
- Black DM, Bilezikian JP, Ensrud KE, et al, for the PaTH Study Investigators. One year of alendronate after one year of parathyroid hormone (1–84) for osteoporosis. N Engl J Med 2005;353:555–565.
- Rittmaster RS, Bolognese M, Ettinger MP, et al. Enhancement of bone mass in osteoporotic women with parathyroid hormone followed by alendronate. J Clin Endocrinol Metab 2000;85:2129–2134.
- Ettinger B, San Martin J, Crans G, Pavo I. Differential effects of teriparatide on BMD after treatment with raloxifene or alendronate. J Bone Miner Res 2004;19:745–751.
- Wells G, Tugwell P, Shea B, et al, for the Osteoporosis Methodology Group and the Osteoporosis Research Advisory Group. Meta-analyses of therapies for postmenopausal osteoporosis. V. Meta-analysis of the efficacy of hormone replacement therapy in treating and preventing osteoporosis in postmenopausal women. Endocr Rev 2002;23:529–539.
- Writing Group for the PEPI. Effects of hormone therapy on bone mineral density: results from the Postmenopausal Estrogen/Progestin Interventions (PEPI) trial. The Writing Group for the PEPI. JAMA 1996;276:1389–1396.
- Cauley JA, Robbins J, Chen Z, et al, for the Women's Health Initiative Investigators. Effects of estrogen plus progestin on risk of fracture and bone mineral density: the Women's Health Initiative randomized trial. JAMA 2003;290:1729–1738.
- Lindsay R, Gallagher JC, Kleerekoper M, Pickar JH. Effect of lower doses of conjugated equine estrogens with and without medroxyprogesterone acetate on bone in early postmenopausal women. JAMA 2002;287:2668–2676.
- Prestwood KM, Kenny AM, Kleppinger A, Kulldorff M. Ultralow-dose micronized 17β-estradiol and bone density and bone metabolism in older women: a randomized controlled trial. JAMA 2003;290:1042–1048.
- Ettinger B, Ensrud KE, Wallace R, et al. Effects of ultralow-dose transdermal estradiol on bone mineral density: a randomized clinical trial. Obstet Gynecol 2004;104:443–451.
- Recker RR, Davies KM, Dowd RM, Heaney RP. The effect of low-dose continuous estrogen and progesterone therapy with calcium and vitamin D on bone in elderly women: a randomized, controlled trial. Ann Intern Med 1999;130:897–904.
- Weiss SR, Ellman H, Dolker M. A randomized controlled trial of four doses of transdermal estradiol for preventing postmenopausal bone loss: Transdermal Estradiol Investigator Group. Obstet Gynecol 1999;94:330–336.
- Al-Azzawi F, Lees B, Thompson J, Stevenson JC. Bone mineral density in postmenopausal women treated with a vaginal ring delivering systemic doses of estradiol acetate. Menopause 2005;12:331–339.
- Grady D, Rubin SM, Petitti DB, et al. Hormone therapy to prevent disease and prolong life in postmenopausal women.Ann Intern Med 1992;117:1016–1037.
- Torgerson DJ, Bell-Syer SE. Hormone replacement therapy and prevention of nonvertebral fractures: a meta-analysis of randomized trials. JAMA 2001;285:2891–2897.
- Banks E, Beral V, Reeves G, Balkwill A, Barnes I, for the Million Women Study Collaborators. Fracture incidence in relation to the pattern of use of hormone therapy in postmenopausal women. JAMA 2004;291:2212–2220.
- Anderson GL, Limacher M, Assaf AR, et al, for the Women's Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA 2004;291:1701–1712.
- Chlebowski RT, Hendrix SL, Langer RD, et al, for the WHI Investigators. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women's Health Initiative Randomized Trial. JAMA2003;289:3243–3253.
- Wassertheil-Smoller S, Hendrix SL, Limacher M, et al, for the WHI Investigators. Effect of estrogen plus progestin on stroke in postmenopausal women: the Women's Health Initiative: a randomized trial. JAMA 2003;289:2673–2684.
- Manson JE, Hsia J, Johnson KC, et al, for the Women's Health Initiative Investigators. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med 2003;349:523–534.
- Cushman M, Kuller LH, Prentice R, et al, for the Women's Health Initiative Investigators. Estrogen plus progestin and risk of venous thrombosis. JAMA 2004;292:1573–1580.
- Shumaker SA, Legault C, Rapp SR, et al, for the WHIMS Investigators. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. JAMA 2003;289:2651–2662.
- The North American Menopause Society. Estrogen and progestogen use in postmenopausal women: July 2008 position statement of The North American Menopause Society. Menopause 2008;15:584–603.
- Gallagher JC, Rapuri PB, Haynatzki G, Detter JR. Effect of discontinuation of estrogen, calcitriol, and the combination of both on bone density and bone markers. J Clin Endocrinol Metab 2002;87:4914–4923.
- Greendale GA, Espeland M, Slone S, Marcus R, Barrett-Connor E, for the PEPI Safety Follow-up Study. Bone mass response to discontinuation of long-term hormone replacement therapy: results from the Postmenopausal Estrogen/Progestin Interventions (PEPI) Safety Follow-up Study. Arch Intern Med 2002;162:665–672.
- Greenspan SL, Emkey RD, Bone HG, et al. Significant differential effects of alendronate, estrogen, or combination therapy on the rate of bone loss after discontinuation of treatment of postmenopausal osteoporosis: a randomized, double-blind, placebo-controlled trial. Ann Intern Med 2002;137:875–883.
- Tremollieres FA, Pouilles JM, Ribot C. Withdrawal of hormone replacement therapy is associated with significant vertebral bone loss in postmenopausal women. Osteoporos Int 2001;12:385–390.
- Barrett-Connor E, Wehren LE, Siris ES, et al. Recency and duration of postmenopausal hormone therapy: effects of bone mineral density and fracture risk in the National Osteoporosis Risk Assessment (NORA) study. Menopause2003;10:412–419.
- Yates J, Barrett-Connor E, Barlas S, Chen YT, Miller PD, Siris ES. Rapid loss of hip fracture protection after estrogen cessation: evidence from the National Osteoporosis Risk Assessment. Obstet Gynecol 2004;103:440–446.
- Silverman SL. Calcitonin. Endocrinol Metab Clin North Am 2003;32:273–284.
- Overgaard K, Hansen MA, Jensen SB, Christiansen C. Effect of salcatonin given intranasally on bone mass and fracture rates in established osteoporosis: a dose-response study. BMJ 1992;305:556–561.
- Chesnut CH III, Silverman S, Andriano K, et al. A randomized trial of nasal spray salmon calcitonin in postmenopausal women with established osteoporosis: the Prevent Recurrence of Osteoporotic Fractures Study. PROOF study group.Am J Med 2000;109:267–276.
- Pun KK, Chan LW. Analgesic effect of intranasal salmon calcitonin in the treatment of osteoporotic vertebral fractures.Clin Ther 1989;11:205–209.
- Lyritis GP, Ioannidis GV, Karachalios T, et al. Analgesic effect of salmon calcitonin suppositories in patients with acute pain due to recent osteoporotic vertebral crush fractures: a prospective double-blind, randomized, placebo-controlled clinical study. Clin J Pain 1999;15:284–289.
- Blau LA, Hoehns JD. Analgesic efficacy of calcitonin for vertebral pain. Ann Pharmacother 2003;37:564–570.
- Bone HG, Greenspan SL, McKeever C, et al. Alendronate and estrogen effect in postmenopausal women with low bone mineral density. Alendronate/Estrogen Study Group. J Clin Endocrinol Metab 2000;85:720–726.
- Harris ST, Eriksen EF, Davidson M, et al. Effect of combined risedronate and hormone replacement therapies on bone mineral density in postmenopausal women. J Clin Endocrinol Metab 2001;86:1890–1897.
- Cummings SR, Ettinger B, Delmas PD, et al, for the LIFT Trial Investigators. The effects of tibolone in older postmenopausal women. N Engl J Med 2008;359:697–708.
- Meunier PJ, Roux C, Seeman E, et al. The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. N Engl J Med 2004;350:459–468.
- Reginster JY, Seeman E, De Vernejoul MC, et al. Strontium ranelate reduces the risk of nonvertebral fractures in postmenopausal women with osteoporosis: treatment of peripheral osteoporosis (TROPOS) study. J Clin Endocrinol Metab 2005;90:2816–2822.
- Greenspan SL, Bone HG, Ettinger MP, et al. Effect of recombinant human parathyroid hormone (1–84) on vertebral fracture and bone mineral density in postmenopausal women with osteoporosis: a randomized trial. Ann Intern Med2007;146:326–339.
- Miller PD, Chines AA, Christiansen C, et al. Effects of bazedoxifene on BMD and bone turnover in postmenopausal women: 2-yr results of a randomized, double-blind, placebo-, and active-controlled study. J Bone Miner Res2008;23:525–535.
- Silverman SL, Christiansen C, Genant HK, et al. Efficacy of bazedoxifene in reducing new vertebral fracture risk in postmenopausal women with osteoporosis: results from a 3-year, randomized, placebo-, and active-controlled clinical trial. J Bone Miner Res 2008;23:1923–1934.
- Cummings SR, Eastell R, Ensrud K, et al. The effects of lasofoxifene on fractures and breast cancer: 3-year results from the PEARL trial. J Bone Miner Res 2008;23(Suppl 1):S81.
- McClung MR, Siris E, Cummings S, et al. Prevention of bone loss in postmenopausal women treated with lasofoxifene compared with raloxifene. Menopause 2006;13:377–386.
- McClung MR, Lewiecki EM, Cohen SB, et al. Denosumab in postmenopausal women with low bone mineral density. N Engl J Med 2006;354:821–831.
- Cummings SR, Martin JS, McClung MR, et al, for the FREEDOM Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 2009;361:756–765.





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 