This Journal feature begins with a case vignette that includes a therapeutic recommendation. A discussion of the clinical problem and the mechanism of benefit of this form of therapy follows. Major clinical studies, the clinical use of this therapy, and potential adverse effects are reviewed. Relevant formal guidelines, if they exist, are presented. The article ends with the author's clinical recommendations.
A 37-year-old woman presented with palpitations, tremulousness, shortness ofbreath, and a 9-kg (20-lb)weight loss, and received a diagnosis of Graves' hyperthyroidism. At the time of diagnosis, she had mild proptosis, no diplopia, and no signs of eye inflammation. Her thyroid gland was two times the normal size and nonnodular. Her initial serum triiodothyronine (T3) concentration was 655 ng per deciliter (9.2 nmol per liter), and her free thyroxine (T4) concentration was 5.7 ng per deciliter (73 pmol per liter). She was treated with methimazole for a year, and her thyroid tests became normal. She discontinued the methimazole 10 weeks before the current presentation with recurrent palpitations and tremulousness. Her serum T3 concentration is 345 ng per deciliter (5.4 nmol per liter), and her free T4 concentration is 2.8 ng per deciliter (36.0 pmol per liter). The patient does not smoke. She has a 3-year-old daughter and wishes to become pregnant again. Her endocrinologist recommends radioiodine ablation of her thyroid.
THE CLINICAL PROBLEM
Hyperthyroidism is predominantly a disorder in women. Overt hyperthyroidism, defined as subnormal serum thyrotropin concentrations with elevated free T3 or free T4 concentrations, has a prevalence of approximately 0.6% among women.1 In a review of several large studies, the incidence of hyperthyroidism was approximately 0.4 cases per 1000 women per year; the incidence in men was 25% or less of the incidence in women.1 Subclinical hyperthyroidism, defined as subnormal levels of serum thyrotropin with normal levels of free T3 and free T4, has a prevalence of 0.7% in the United States.2 Graves' disease is the most common cause of hyperthyroidism in all age groups in the United States,3 but for areas in which the population has iodine deficiency, the prevalence of toxic adenoma and multinodular goiter increases with age, and these disorders are more common than Graves' disease in older persons. 4
Untreated hyperthyroidism may lead to cardiovascular disease, including atrial fibrillation, cardiomyopathy, and congestive heart failure.5 Severe thyrotoxicosis (or thyroid storm) is associated with a mortality of 20 to 50%.6 Increased bone turnover occurs with untreated hyperthyroidism, leading to osteoporosis and fracture.7
PATHOPHYSIOLOGY AND THE EFFECT OF THERAPY
Thyroid hormone acts by binding to specific thyroid hormone receptors, which interact with thyroid hormone response elements on multiple genes, modifying gene transcription in virtually all tissues. Many nongenomic actions have also been described.8 An increase in the expression of beta-adrenergic receptors and cyclic AMP production, differential expression of cyclic AMP isoforms, and a reduction in the expression of inhibitory G-protein subunits, among other mechanisms, all contribute to increased thermogenesis.9 In addition to its effect on cardiac and skeletal health, hyperthyroidism has adverse effects on virtually all organ systems. The hyperadrenergic state leads to agitation and insomnia; dyspnea may occur because of increased oxygen consumption, anemia, and weakness in respiratory muscles; increased gut motility results in hyperdefecation; and myopathy results in proximal muscle weakness.
Graves' disease is an autoimmune disorder caused by an antibody that acts as an agonist on the thyrotropin receptor. The result is unregulated synthesis of thyroid hormone. Spontaneous remission occurs in approximately 30% of patients.10 Ophthalmopathy characterized by inflammation of periorbital and retro-orbital connective tissue, fat, and muscle is clinically evident in approximately one third of patients and is unique to Graves' disease. Thyrotropin receptors are found on orbital fibroblasts and adipocytes; they are probably the autoimmune target responsible for Graves' ophthalmopathy.11
Toxic adenoma and multinodular goiter cause autonomous, unregulated synthesis of thyroid hormone. In some cases, the cause is a mutation in the thyrotropin receptor gene, which results in constitutive activation.12 Toxic adenoma and multinodular goiter are not associated with ophthalmopathy; these conditions do not resolve spontaneously unless the hyperthyroidism was precipitated by administration of pharmacologic amounts of iodine or, in rare cases, by the spontaneous infarction of a toxic adenoma.
Iodine is a substrate for thyroid hormone synthesis and is actively transported into the thyroid follicular cells by an iodine symporter. It is oxidized and covalently bound to the tyrosyl residues of thyroglobulin. Coupling of iodinated tyrosyl residues results in the formation of T4 and T3, which are stored within the colloid space (Figure 1A).
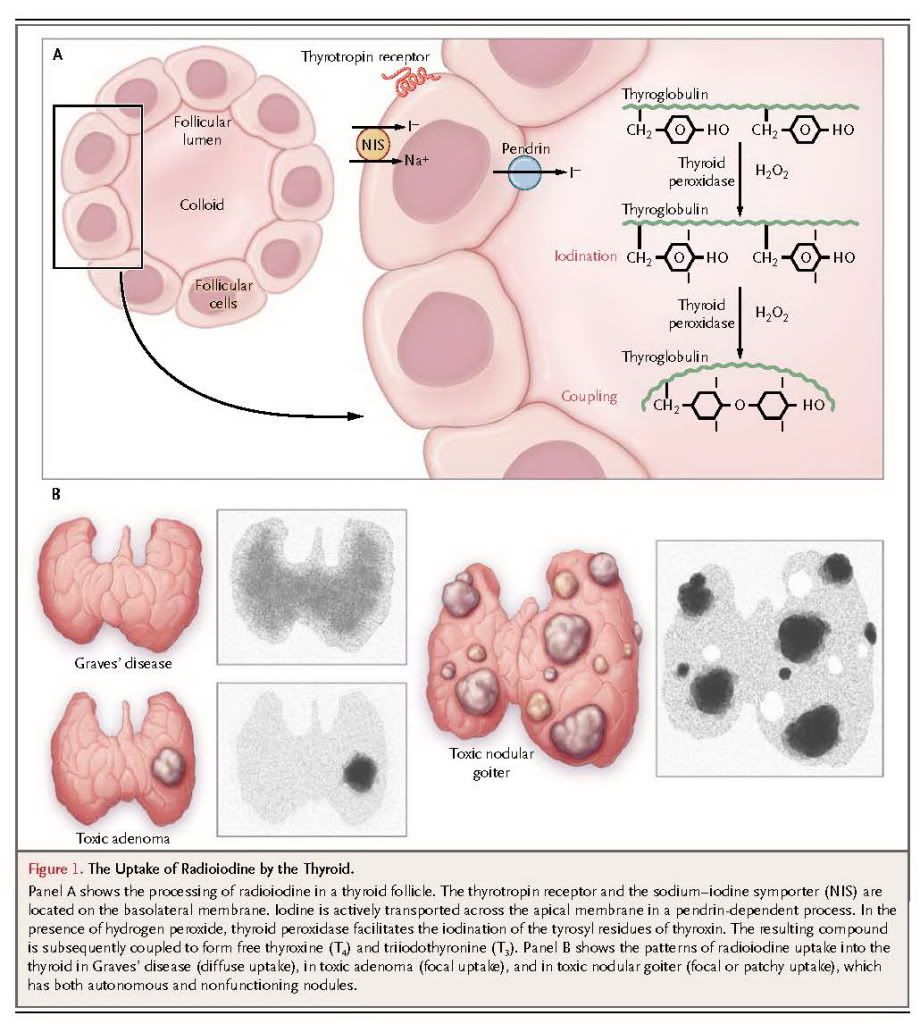
Radioiodine (131I) is similarly processed, and its beta emissions result in tissue necrosis,13 effectively ablating functional thyroid tissue over the course of 6 to 18 weeks or more.
In normal thyroid tissue, thyrotropin is required to stimulate iodine uptake into follicular cells. Since thyrotropin-receptor antibodies activate the thyrotropin receptors in Graves' disease, radioiodine is concentrated within the entire gland. In contrast, in toxic adenoma or toxic nodular goiter, thyrotropin is suppressed by the hyperthyroid state, and radioiodine is concentrated only in tissue that is autonomous (i.e., not subject to normal regulation by thyrotropin) (Figure 1B). However, if the patient is treated with antithyroid drugs before radioiodine administration and if thyrotropin levels are allowed to become normal or high, then radioiodine will be concentrated in both the autonomous and normal thyroid tissue.
CLINICAL EVIDENCE
Radioiodine has been used for the treatment of Graves' hyperthyroidism since the 1940s, and conclusions regarding its efficacy are based primarily on extensive clinical experience rather than randomized trials. One randomized, controlled trial involving 179 patients with Graves' hyperthyroidism compared the effects of radioiodine with those of surgery and antithyroid drugs14-17; patients under 35 years of age were not enrolled in the radioiodine group. Of the 71 patients receiving 18 months of treatment with methimazole, 16% had adverse reactions, 6% had an inadequate response to therapy, and 37% had a relapse. Of the 67 patients who underwent surgery, none had complications, and 6% had a relapse. Hypothyroidism developed in 39 of the 41 patients in the radioiodine group; the other 2 patients declined the assigned treatment.14 The average first dose of radioiodine was relatively low, at 6.8 mCi (252 MBq), and 18 of the patients being treated required more than one dose. This trial showed that ophthalmopathy worsened in patients treated with radioiodine as compared with patients receiving the alternative therapies: ophthalmopathy worsened in 10% of the patients receiving methimazole, 16% of those undergoing surgery, and 33% of those treated with radioiodine.15 However, patient satisfaction with all three forms of therapy was equivalent after treatment16 and 14 to 21 years later.17
CLINICAL USE
Three efficacious treatments are available for hyperthyroidism. Radioiodine therapy and surgery are considered definitive therapies, since the primary goal of this approach to treatment is to destroy hyperfunctioning thyroid tissue. Antithyroid drugs (methimazole, carbimazole, and propylthiouracil) are used in one of two ways. On occasion before radioiodine administration and usually before surgery, several weeks of treatment with an antithyroid drug is administered to achieve a euthyroid state. Antithyroid drugs are also used in Graves' hyperthyroidism for 1 to 2 years, or longer, with the hope of achieving a remission.18 Remission of hyperthyroidism is not expected when antithyroid drugs are used during the long-term treatment of toxic adenoma or toxic multinodular goiter; in these cases, early definitive therapy with radioiodine or surgery is indicated.
The choice of therapy involves multiple considerations (Table1)
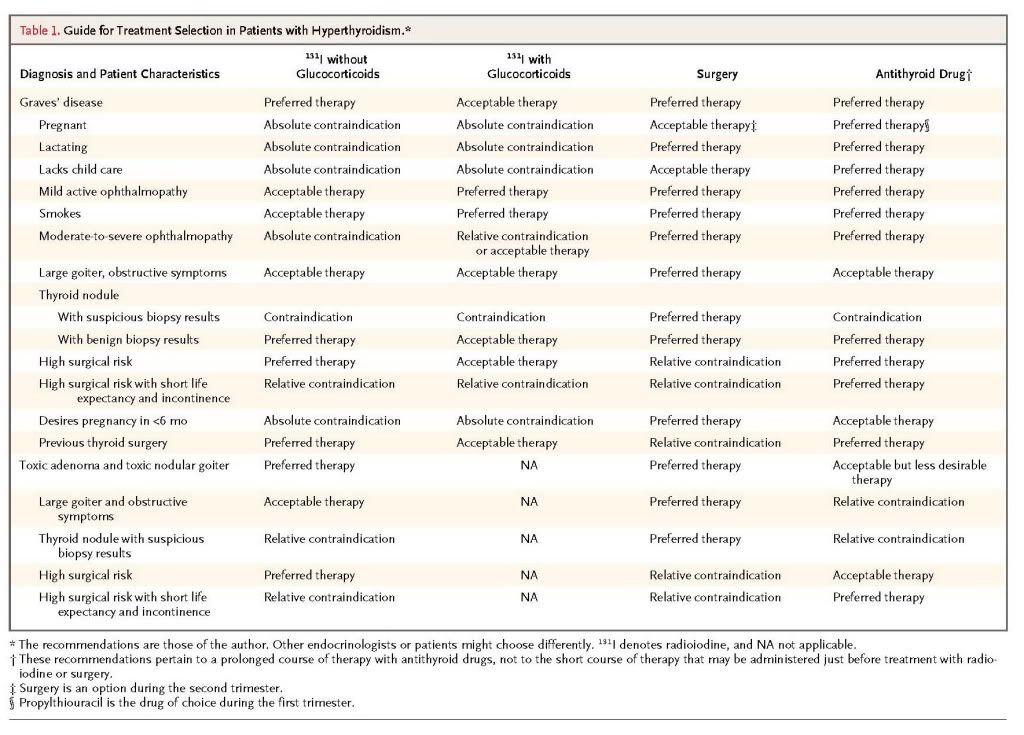
An endocrinologist should carefully review with the patient each treatment's benefits and risks and make a specific recommendation; ultimately, however, the decision must respect the patient's reasonable preferences. A 1991 survey of members of the thyroid associations in America, Europe, and Japan showed that use of radioiodine is most popular in North America, with 69% of physicians recommending it as first-line therapy for a typical case of Graves' disease, as compared with 22% of physicians in Europe and 11% of those in Japan.19 The reasons for these differences are unknown.
Absolute contraindications to radioiodine treatment are pregnancy, lactation, and an inability to comply with radiation safety regulations. Radioiodine is considered safe for use in women of childbearing age and in older children.20 Patients who are allergic to iodinated radiocontrast agents are usually not allergic to radioiodine.
Moderately severe ophthalmopathy may be a contraindication to treatment with radioiodine, since radioiodine may exacerbate the condition. 15,21,22 Worsening ophthalmopathy is more common in cigarette smokers than in nonsmokers.21 Concurrent administration of glucocorticoids mitigates exacerbations, at least in patients with mild ophthalmopathy.22-24
Beta-adrenergic blocking agents, if not contraindicated, are usually administered when hyperthyroidism is diagnosed in order to ameliorate symptoms. Most endocrinologists also treat high-risk patients with antithyroid drugs for several weeks before administering radioiodine in an attempt to achieve normal or near-normal thyroid function as rapidly as possible.25 High-risk patients include elderly persons, patients with underlying cardiovascular disease, and patients with severe hyperthyroid symptoms or with concentrations of thyroid hormone that are two to three times as high as the upper limit of the normal range. Pretreatment with an antithyroid drug may increase the risk of treatment failure with the initial radioiodine dose25; in one study, this occurred after the administration of propylthiouracil but not methimazole.26 The use of higher doses of radioiodine can compensate for this effect.25 Antithyroid drugs are discontinued 2 to 3 days before the administration of radioiodine. 27
Radioiodine is given orally as a single dose of 131I-labeled sodium iodide (Na131I) in liquid or capsule form. One randomized trial suggested the superiority of calculated-dose regimens,28 which take into account the estimated thyroid weight in grams, the desired dose (100 to 200 μCi per gram), and the 24-hour radioiodine uptake. Another study, however, reported that the use of three fixed doses in amounts based on gland size as determined by palpation (5, 10, or 15 mCi [185, 370, or 555 MBq]) was as effective.29 I generally prefer the use of the calculated-dose approach, since the radioiodine uptake test can also be used to help determine the cause of the hyperthyroidism. Iodine is rapidly absorbed, is concentrated in the thyroid, and is cleared by the kidneys or through hemodialysis. Clearance of radioiodine is reduced in patients with renal failure, which means that body organs and bone marrow are effectively exposed to higher doses.30
The cell necrosis induced by radioiodine occurs gradually, and an interval of 6 to 18 weeks or longer must elapse before a hypothyroid or euthyroid state is achieved. During that interval, hyperthyroidism may transiently worsen before it resolves. If the patient was pretreated with antithyroid drugs, they may be resumed 3 to 7 days after radioiodine administration, and treatment with beta-adrenergic blocking agents is typically continued. The patient's thyroid function is monitored at intervals of 4 to 6 weeks. When thyroid function has normalized, treatment with beta-blockers and antithyroid drugs is stopped and levothyroxine is administered as indicated, with the goal of maintaining normal thyroid function. Suppression of serum thyrotropin may be prolonged after successful treatment; therefore measurement of free T4 and T3 is essential for several months after radioiodine therapy.31
If sufficient radioiodine is administered, hypothyroidism develops in 80 to 90% of patients with Graves' disease; 14% of patients require additional treatment.32 Failure of the first dose to cure hyperthyroidism and the need for a second course of treatment may be obvious after an interval as short as 3 months, if the gland size fails to regress and thyroid hormone levels remain quite high. However, if the degree of hyperthyroidism is minimal 3 months after the initial treatment, hormone levels may slowly approach the normal or hypothyroid range, obviating the need for retreatment. Attainment of normal thyroid function after a single dose of radioiodine is an elusive goal. Continued stimulation of thyrotropin receptors in nonablated tissue may cause recurrent hyperthyroidism. More commonly, hypothyroidism occurs well after treatment has concluded.33 In patients with Graves' disease, complete ablation of the gland is the goal of radioiodine treatment.
In patients with toxic adenoma, the goal of therapy is to ablate only the adenoma. If radioiodine is administered when the thyrotropin level is suppressed as a result of the patient's hyperthyroid state, it does not become concentrated in normal extranodular tissue and the patient should be euthyroid after ablation of the adenoma.34 However, hypothyroidism is likely if the patient is pretreated with antithyroid drugs and thyrotropin levels are not suppressed at the time of treatment,35 allowing radioiodine to become concentrated in normal thyroid epithelium. In patients with toxic nodular goiter, the outcome depends on the extent of autonomous thyroid tissue and the thyrotropin level at the time of treatment. However, another goal may be to reduce goiter size: normal or elevated levels of thyrotropin facilitate iodine uptake into both autonomous and nonautonomous thyroid tissue, reducing goiter size but increasing the risk of hypothyroidism.
Retreatment with radioiodine is necessary in 10 to 30% of patients with toxic adenoma36 and in 6 to 18% of patients with toxic nodular goiter.37 Hypothyroidism occurs in only 3% of patients after 1 year of treatment but is present in 24 to 60% of patients after 20 to 24 years, and it occurs more commonly in patients who also have Hashimoto's thyroiditis.38,39 Radioiodine reduces goiter size by 40%37 and improves compressive symptoms in slightly less than 50% of patients.40
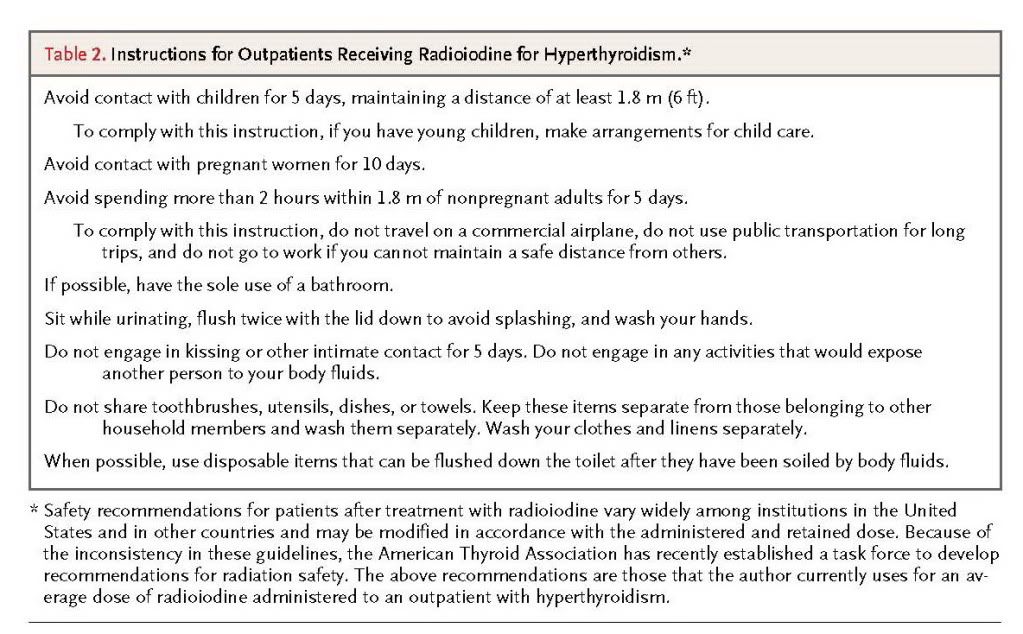
In the United States, radioiodine is administered on an outpatient basis, but some countries, depending on the dose, require several days of hospitalization because of radiation safety concerns. Currently, radiation safety requirements vary from state to state and more markedly from country to country, and they may depend on the administered or retained radiation dose. Typically, patients are advised to avoid contact with children and pregnant women for up to a week or longer, to restrict close contact with nonpregnant adults for several days (e.g., a 2-hour limit for contact at 1.8 m [6 ft] or closer), and to be meticulous about toilet use and cleaning (Table2)
Administration of an average dose of radioiodine costs $392 to $750. In one analysis, the overall cost of treatment for Graves' hyperthyroidism, taking into account laboratory testing, imaging, office visits, and possible complications, was $23,610 for radioiodine and $33,195 for surgery, according to 2007 Medicare rates.41 Additional costs to the patient may be incurred as a result of missed work or the need to provide child care because of radiation safety requirements.
ADVERSE EFFECTS
Radiation thyroiditis, a painful inflammation of the thyroid, occurs in 1% of patients and lasts a few weeks. This condition is frequently associated with exacerbation of thyrotoxicosis because of the destruction-mediated release of thyroid hormone. Treatment typically consists of nonsteroidal antiinflammatory drugs and beta-adrenergic blockers, but some patients require glucocorticoids to relieve the pain.
In up to 5% of patients with toxic nodular goiter, Graves' disease develops after radioiodine therapy.42 It is likely that the release of thyroid antigens from inflamed tissue triggers the production of thyrotropin-receptor antibody. This condition is treated with a second dose of radioiodine.
In most studies, radioiodine has not been associated with an increased risk of cancer. The Cooperative Thyrotoxicosis Therapy Follow-up Study Group has followed 35,593 patients after treatment for hyperthyroidism, and after a mean follow-up of 21 years, the group reported no increase in the overall risk of death from cancer among patients who received radioiodine.43 There was a small increase in the risk of thyroid cancer, primarily among patients with toxic nodular goiter, which was thought to be partly due to the underlying disease rather than the treatment. In another study, which for 36 years followed 107 patients with hyperthyroidism who had been treated before the age of 20 years, no increased risk of cancer was reported.20 A British study of 7417 patients reported a reduced risk of cancer overall but a slight increase in the risk of thyroid and colon cancer.44 A Finnish study of 2793 patients reported an increased risk of stomach, kidney, and breast cancer.45
Patients receiving radioiodine therapy are at increased risk for death from cardiovascular disease primarily in the first year after treatment. This is thought to be a consequence of the thyrotoxic state rather than the treatment.46
Nodular thyroid tissue may regress after radioiodine ablation, but it rarely disappears, and over time calcifications may develop. Calcified nodules are evident on subsequent imaging and may be viewed as possibly precancerous by radiologists, which may lead to thyroid surgery.
AREAS OF UNCERTAINTY
There is considerable uncertainty about the optimal therapy for hyperthyroidism, owing to the absence of data from large, well-designed, randomized, controlled trials. Moreover, the perception of what constitutes optimal therapy appears to differ dramatically among physicians, patients, and professional societies, being highly dependent on the relative values placed on risks and outcomes. Questions that could be addressed by well-designed studies include the following: Which patients receiving radioiodine require pretreatment with antithyroid drugs? When patients are first treated with antithyroid drugs, should the drugs be reinstated after treatment with radioiodine, and if so, at what interval? What are the optimal subsequent interventions, and what are the costs of follow-up after radioiodine therapy for patients with toxic nodular goiter?
Controversy also surrounds guidelines for radiation safety after radioiodine therapy. For example, in 2008, the Nuclear Regulatory Commission released a statement regarding the protection of children who may come into contact with patients treated with radioiodine. In this statement, it was suggested that physicians should consider keeping patients hospitalized if they live with infants or young children.47 Some experts argued that this recommendation, although not a mandate, would result in unnecessarily restrictive management of patient care.48 Uncertainty about this and many other issues related to the safe and appropriate use of radioiodine persist because there are few direct data on which to base firm recommendations.
GUIDELINES FROM PROFESSIONAL SOCIETIES
The draft version of the guidelines on the management of hyperthyroidism from the American Thyroid Association and the American Association of Clinical Endocrinologists, expected to be published in final form in 2011, states that radioiodine, antithyroid drugs, and surgery are all reasonable treatment options for hyperthyroidism (Bahn RS: personal communication). These guidelines emphasize the importance of detailed discussions with the patient regarding each option's benefits and risks relative to coexisting conditions, lifestyle, and the patient's values. Pretreatment with antithyroid drugs should be considered in elderly persons and in patients with underlying cardiovascular disease, severe hyperthyroid symptoms, or thyroid hormone concentrations that are two to three times the upper limit of the normal range. Surgery, rather than radioiodine therapy, is recommended for patients with active, moderately severe Graves' ophthalmopathy. Concurrent use of glucocorticoids should be considered in those with active, mild ophthalmopathy and in smokers. Children are usually treated with methimazole for 1 to 2 years, but treatment with radioiodine, surgery, or methimazole is appropriate for children over 10 years of age.





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 