Pei Chen, Ph.D., Juei-Jueng Lin, M.D., Chin-Song Lu, M.D., Cheung-Ter Ong, M.D., Peiyuan F. Hsieh, M.D., Chih-Chao Yang, M.D., Chih-Ta Tai, M.D., Shey-Lin Wu, M.D., Cheng-Hsien Lu, M.D., Yung-Chu Hsu, M.D., Hsiang-Yu Yu, M.D., Long-Sun Ro, M.D., Chung-Ta Lu, M.D., Chun-Che Chu, M.D., Jing-Jane Tsai, M.D., Yu-Hsiang Su, M.D., Sheng-Hsing Lan, M.D., Sheng-Feng Sung, M.D., Shu-Yi Lin, M.S., Hui-Ping Chuang, B.S., Li-Chen Huang, B.S., Ying-Ju Chen, M.S., Pei-Joung Tsai, M.S., Hung-Ting Liao, M.S., Yu-Hsuan Lin, M.S., Chien-Hsiun Chen, Ph.D., Wen-Hung Chung, M.D., Ph.D., Shuen-Iu Hung, Ph.D., Jer-Yuarn Wu, Ph.D., Chi-Feng Chang, Ph.D., Luke Chen, Ph.D., Yuan-Tsong Chen, M.D., Ph.D., and Chen-Yang Shen, Ph.D. for the Taiwan SJS Consortium
Background
Carbamazepine, an anticonvulsant and a mood-stabilizing drug, is the main cause of the Stevens–Johnson syndrome (SJS) and its related disease, toxic epidermal necrolysis (TEN), in Southeast Asian countries. Carbamazepine-induced SJS–TEN is strongly associated with the HLA-B*1502 allele. We sought to prevent carbamazepine-induced SJS–TEN by using HLA-B*1502 screening to prospectively identify subjects at genetic risk for the condition.
Methods
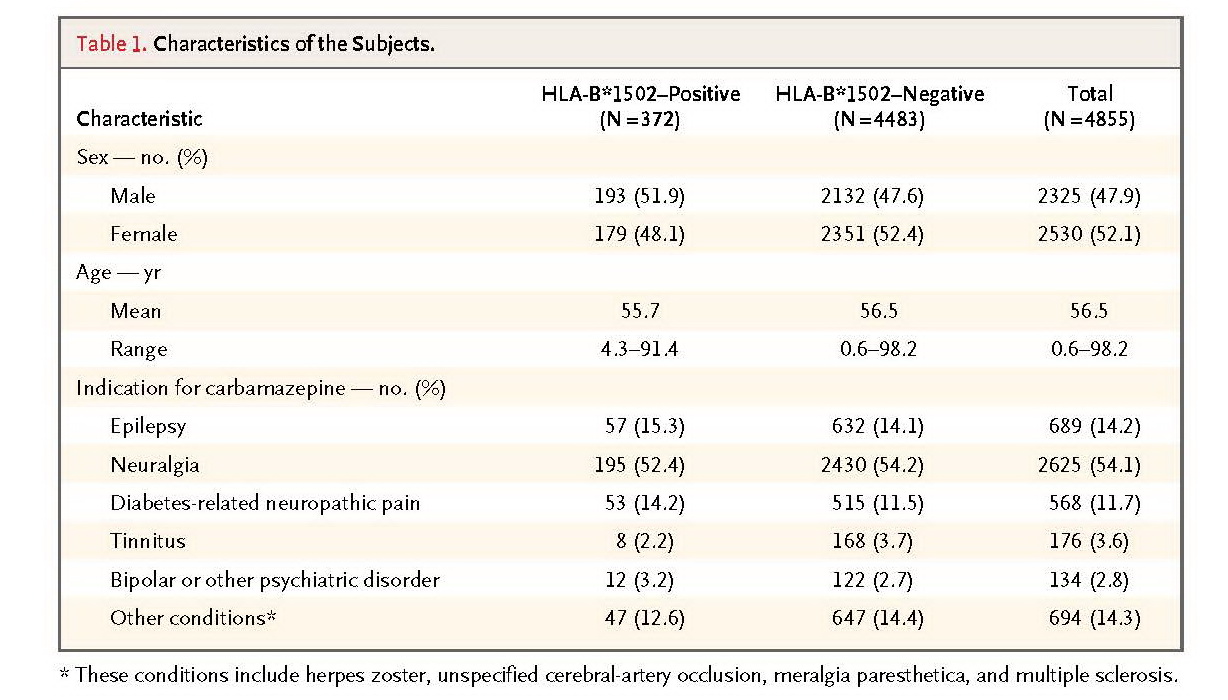
From 23 hospitals in Taiwan, we recruited 4877 candidate subjects who had not taken carbamazepine. We genotyped DNA purified from the subjects' peripheral blood to determine whether they carried the HLA-B*1502 allele. Those testing positive for HLA-B*1502 (7.7% of the total) were advised not to take carbamazepine and were given an alternative medication or advised to continue taking their prestudy medication; those testing negative (92.3%) were advised to take carbamazepine. We interviewed the subjects by telephone once a week for 2 months to monitor them for symptoms. We used the estimated historical incidence of SJS–TEN as a control.
Results
Mild, transient rash developed in 4.3% of subjects; more widespread rash developed in 0.1% of subjects, who were hospitalized. SJS–TEN did not develop in any of the HLA-B*1502–negative subjects receiving carbamazepine. In contrast, the estimated historical incidence of carbamazepine-induced SJS–TEN (0.23%) would translate into approximately 10 cases among study subjects (P<0.001).
Conclusions
The identification of subjects carrying the HLA-B*1502 allele and the avoidance of carbamazepine therapy in these subjects was strongly associated with a decrease in the incidence of carbamazepine-induced SJS–TEN. (Funded by the National Science Council of Taiwan and the Taiwan Drug Relief Foundation.)








 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 