Raffaele De Caterina, M.D., Ph.D.
N Engl J Med 2011; 364:2439-2450June 23, 2011
Cardiovascular disease is the leading cause of death worldwide, and preventive approaches, particularly achievable dietary changes, have major public health implications. An increased dietary intake of n–3 (polyunsaturated) fatty acids is one such dietary approach. This review discusses advances since the topic was last reviewed in the Journal 1 and highlights current gaps in knowledge.
BIOSYNTHESIS OF ESSENTIAL POLYUNSATURATED FATTY ACIDS
Polyunsaturated fatty acids, organic acids that naturally contain more than one double bond in the aliphatic chain, are named according to the number (>1), position, and configuration of such double bonds, which also largely determine their physical and biologic properties. Biologically relevant families of polyunsaturated fatty acids are the n–6 and the n–3 fatty acids
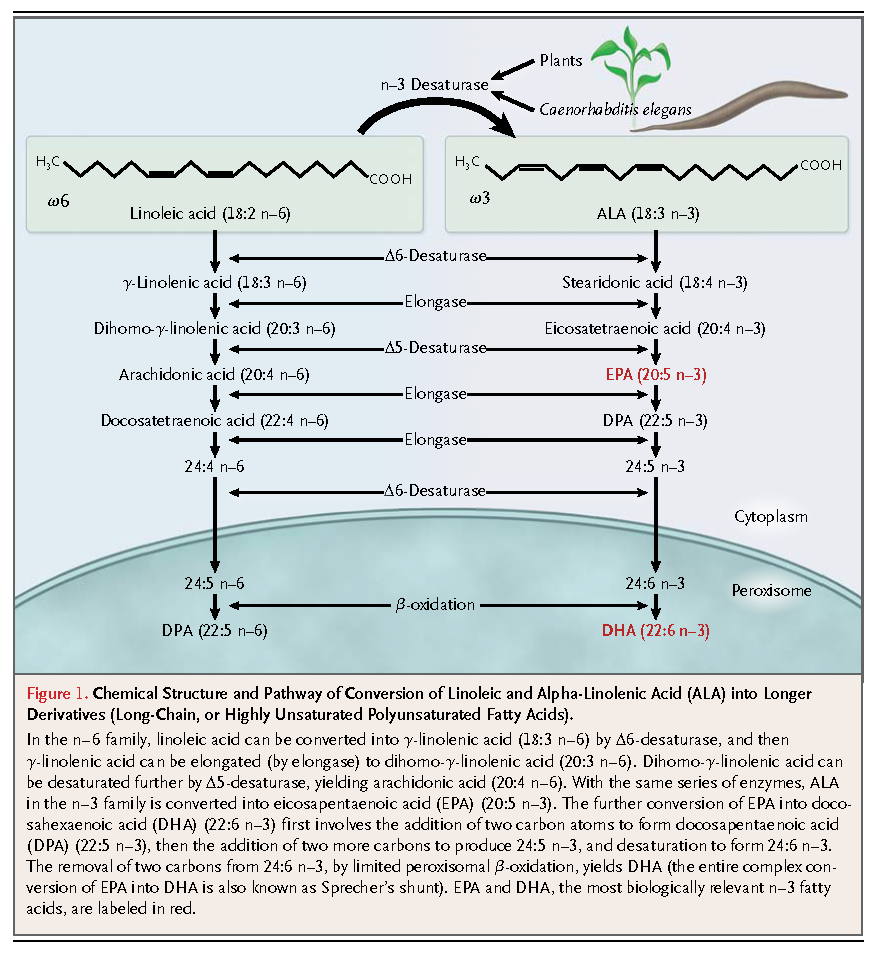
Mammals lack enzymes to insert the double bond in the n–6 or n–3 position; therefore, linoleic acid and alpha-linolenic acid (ALA), as well as some of their elongation products, are essential nutrients for mammals; the lack of these nutrients leads to a syndrome of deficiency of essential fatty acids.6,7 In humans, this deficiency is usually characterized by desquamative rashes and hyperkeratotic dermatoses.8 Current estimates of the minimum requirements for n–6 and n–3 fatty acids in adults are 1.0% and 0.2% of daily energy intake, respectively,9 with acceptable (but not necessarily ideal) macronutrient distribution ranges for eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) of 0.25 to 2.0 g per day.10
Plant tissues and oils are good sources of linoleic acid and ALA. Photosynthesizing plants are especially rich in ALA, accounting for up to 55% of the fatty acids present in green vegetables. However, green vegetables generally contribute little to the optimal intake of ALA in humans, as compared with certain plant oils such as soybean, flaxseed, linseed, and rapeseed (canola) oils, as well as walnuts. Mammalian cells cannot synthesize linoleic acid and ALA, though limited (1 to 5%)11conversion to longer-chain polyunsaturated fatty acids occurs through further desaturation and elongation. Linoleic acid can ultimately be converted to arachidonic acid (20:4 n–6) and, through the same series of enzymes, ALA can be converted to EPA (20:5 n–3). The further conversion of EPA to DHA (22:6 n–3) involves a complex series of additional reactions,12 but the conversion is very limited11 (Figure 1). DHA accumulates in all tissues, including the heart and the vessel wall, but it particularly accumulates in the nervous system and the retina, where it serves important physiological functions.
EPA and DHA enter the food chain through marine phytoplankton, and they pass through fish and marine mammals — seals, walruses, and whales — which are the main components of the Eskimo diet. In Western diets, the main source of EPA and DHA intake is fish, especially oily fish (e.g., mackerel, trout, salmon, herring, and sardines). Fish-oil preparations contain variable amounts of n–3 fatty acids in the form of triglycerides, ethyl esters, or free fatty acids.
Transgenic animals that express the fat-1 gene from the worm Caenorhabditis elegans have been raised. This gene encodes an n–3 fatty acyl desaturase catalyzing the conversion of 18- and 20-carbon n–6 substrates into n–3 fatty acids. A supplementation approach with the use of meat from these transgenic animals might permit a marked enrichment of mammalian cells with n–3 fatty acids beyond that which is possible through dietary approaches, with obvious clinical implications.
METABOLISM AND MECHANISMS OF ACTION
Polyunsaturated fatty acids modulate local signaling and structure, primarily after esterification (prevalently in the sn-2 position) in glycerophospholipids and incorporation into cell membranes (see the Supplementary Appendix, available with the full text of this article at NEJM.org). There are three broad categories of biologic effects: those mediated by the release of bioactive mediators, direct effects on ion channels that modulate a number of events (e.g., arrhythmogenesis), and other direct effects on membranes that require incorporation into cell phospholipids.
The n–3 fatty acids can be released through the actions of phospholipase A2, induced by a variety of stimuli, and are converted through both orthodox and novel pathways to signaling molecules. The orthodox pathways involve reactions catalyzed by cyclooxygenases and lipoxygenases to biologically active eicosanoids, including prostaglandins, thromboxanes, and leukotrienes (Figure 2).
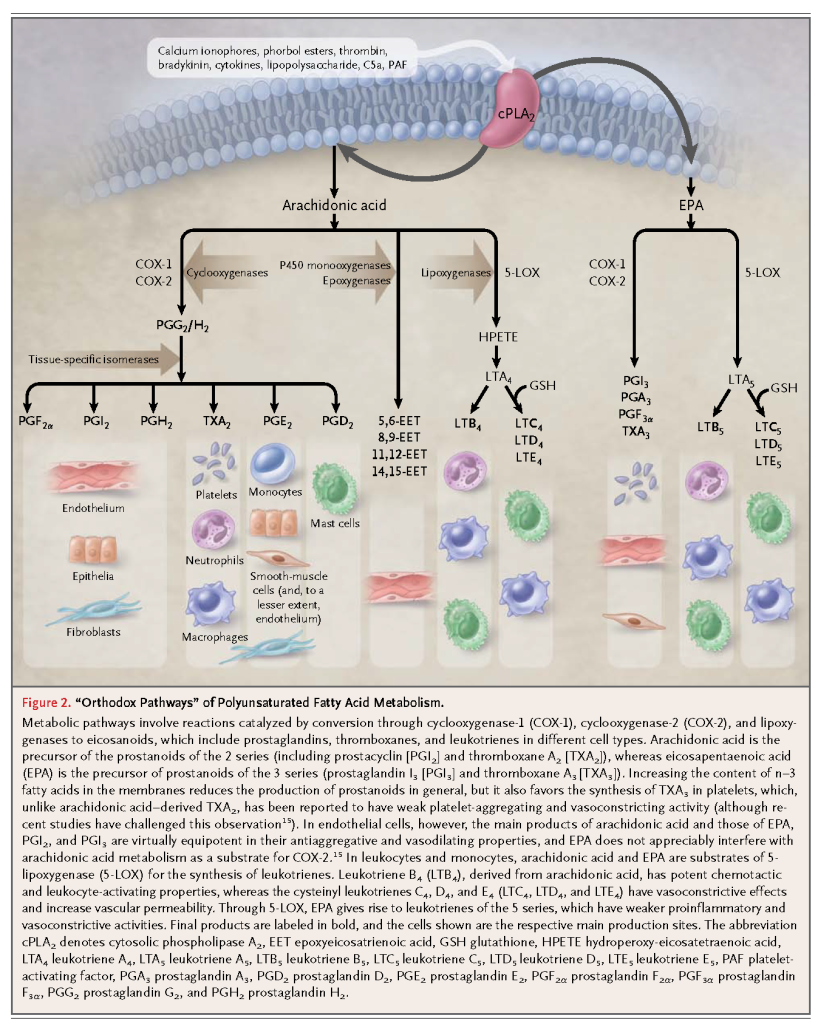
Thromboxanes and leukotrienes derived from n–3 fatty acids are in general much less potent local mediators than the corresponding n–6 fatty acid derivatives in orthodox pathways. The n–3 fatty acids are also converted to biologically active epoxides, alcohols, diols, and ketones through recently discovered new pathways (Figure 3
). A number of local mediators, discovered as a result of elucidating such new pathways, are potent (in the nanomolar range) and stereoselective, with antiinflammatory actions and actions prompting the resolution of inflammation, and they are produced during this resolution phase of inflammation through transcellular biosynthetic routes (i.e., the product of one cell is transformed by neighboring cells). Thus, these compounds are termed “resolution-phase interaction products” (resolvins)16,17 (Figure 3). In addition, unsaturated fatty acids, through nitration, can generate nitro-fatty acids that globally suppress inflammation at micromolar concentrations18 (Figure 3).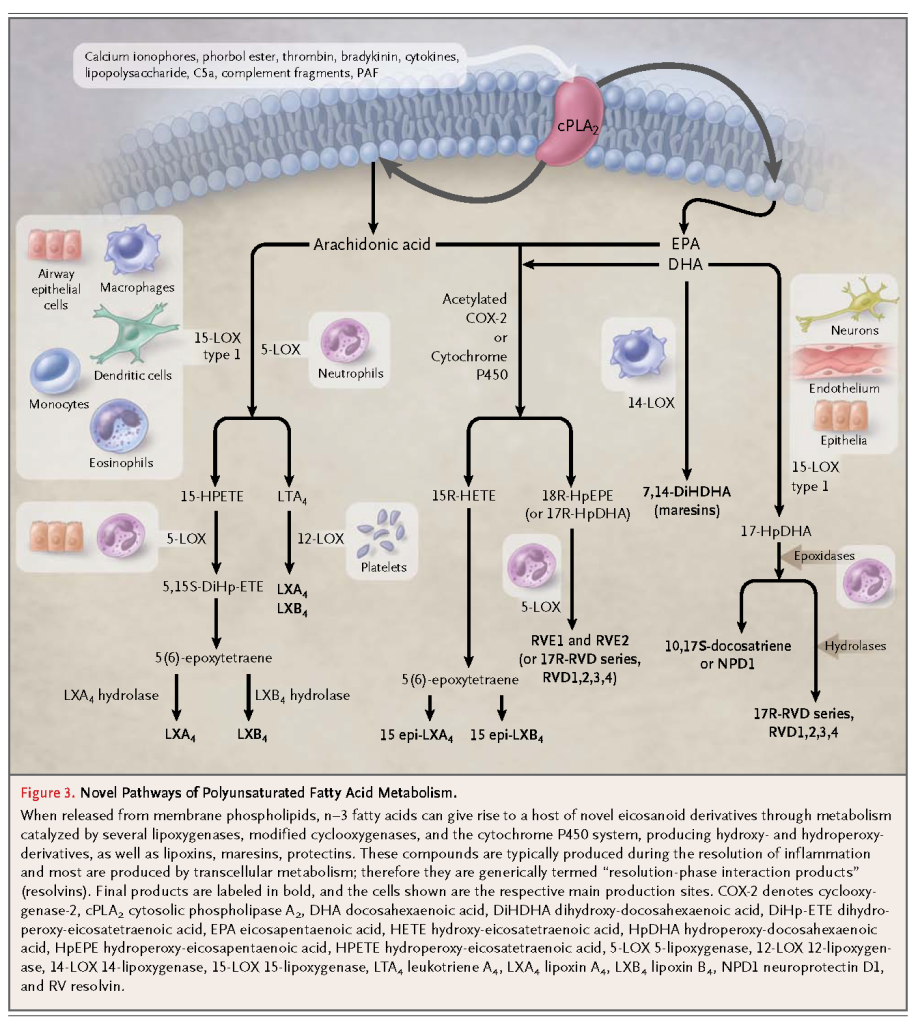
In low micromolar concentrations, n–3 fatty acids have direct effects without undergoing metabolism. Some direct effects, such as antiarrhythmic effects, occur rapidly,19 without incorporation of n–3 fatty acids into the cell membranes. These free fatty acids cause steric interference with sodium, potassium, and calcium channels; this blocking mechanism is distinct from that of other known classes of antiarrhythmic agents.20 Conversely, incorporation into cell membranes is required for slow-onset and long-acting direct antiinflammatory, antiatherogenic effects, modulating the expression of endothelial proinflammatory, proatherogenic genes (genomic effects).21,22 The incorporation of fatty acids appears to alter the properties of lipid rafts and caveolae, contributing to membrane fluidity. Thus, hormone-receptor binding and the function of membrane-associated proteins are affected.23,24 In turn, this incorporation has been associated with decreased generation of intracellular reactive oxygen species and a consequent diminished activation of redox-sensitive transcription factors, such as the nuclear factor-κB system, modifying the expression of proinflammatory, proatherogenic genes.22,25 There is evidence that n–3 fatty acids signal through G-protein–coupled receptor 120, modulating inflammatory and insulin-sensitizing effects that occur in monocytes and macrophages.26
Through one or more of the above mechanisms, n–3 fatty acids may affect several intermediate determinants of cardiovascular risk. At doses of 3 g per day or more, these substances generally reduce hypertriglyceridemia in humans27,28 without changing cholesterol levels substantially.29 The n–3 fatty acids are also associated with decreased levels of markers and mediators of inflammation such as the cytokines interleukin-1β and tumor necrosis factor α.30,31 Studies have shown that increased intake of n–3 fatty acids is associated with a small reduction in blood pressure (approximately 2 to 3 mm Hg systolic and 1 to 2 mm Hg diastolic)32 and with a reduction in the resting heart rate (approximately 3 beats per minute).33 Other studies suggest that the intake of n–3 fatty acids is associated with improved cardiac diastolic filling,33 modulation of autonomic function (thus increasing heart-rate variability),34 and increased baroreceptor control,35 which in turn reduce the risk of fatal arrhythmias. Some data suggest that n–3 fatty acids improve insulin sensitivity36and mildly inhibit platelet function,37,38 though the overall effects on hemostasis appear to be modest. Despite a mild increase in the bleeding time, clinically important bleeding, originally described in Eskimos,4 has not been substantiated in other groups.39,40 A short course of n–3 fatty acid supplementation has been reported to improve endothelial function41 and to reduce features of inflammatory atherosclerotic plaque.
想多了解一些魚油:可以溶解保麗龍的魚油才是好魚油?





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 