Special Considerations in Elderly Patients With Chronic Obstructive Pulmonary Disease
Abstract and Introduction
Purpose. Special considerations in
the selection of medication inhaler devices for elderly patients with
chronic obstructive pulmonary disease (COPD) in the ambulatory care
setting are reviewed.
Summary. Substantial deficiencies in inhaler device technique and
medication adherence are evident in patients with COPD, leading to
suboptimal health outcomes. As the prevalence of COPD rises with age,
elderly patients pose special challenges with regard to inhaler device
selection. In elderly patients with sufficient cognitive function,
manual dexterity, and hand strength, the most influential factors in
inhaler selection are cost reimbursement, device availability, device
convenience, and patient preference. Cost reimbursement may be a
deciding factor in device selection, as nearly all elderly patients are
Medicare beneficiaries. Nebulizers provide a cost-effective alternative
to pressurized metered-dose inhaler (pMDI) and dry powder inhaler (DPI)
devices. DPI device availability is limited to "controller" medications,
while pMDI devices and nebulizers provide complete symptomatic
coverage. Multiple-dose DPIs offer the convenience of rapid medication
administration, ease of handling, and integral dose counters. Given the
availability and expenses of medication devices, ambulatory patients may
prefer combining the convenience of a hand-held inhaler (i.e., pMDI) as
a rescue medication during the active hours of midday with the cost
savings of a nebulized controller medication in the morning and at
night.
Conclusion. In elderly patients with sufficient cognitive
function, manual dexterity, and hand strength, the most important
factors in inhaler device selection are cost reimbursement issues,
device availability, device convenience, and patient preference.
Pharmacist knowledge of appropriate inhaler technique, competent patient
education and demonstration, and follow-up assessment are instrumental
in optimizing device competency and medication adherence.
Introduction
Chronic obstructive pulmonary disease (COPD) is an insidious, progressive syndrome of airway limitation with symptoms often evident only after deterioration to severe disease.[1,2] Consequently, most patients are not diagnosed and treated until advanced stages of the disease. By this time, 85% of patients with COPD develop some degree of activity limitation, while 40% of patients report being unable to work.[3] Advanced stages of COPD are also accompanied by an increasing frequency and severity of disease exacerbations, which are the leading cause of hospitalization and mortality.[1] As of 2008, COPD ranked as the third leading cause of death in the United States after heart disease and cancer.[4] In contrast to heart disease and stroke, mortality rates for COPD increased steadily from 1950 to 2007.[3,4] The goals of therapy for COPD are to improve symptoms (e.g., exercise tolerance) and to reduce the occurrence and intensity of exacerbations.
The cornerstone of treatment for COPD involves aerosol administration of medications through inhaler devices.[1] Currently, the major categories of aerosol delivery devices are pressurized metered-dose inhalers (pMDIs) with or without a spacer, dry powder inhalers (DPIs), and nebulizers. Evidence suggests that any inhaler device category can be equally effective in treating patients.[5,6] However, each device exhibits distinct properties that warrant consideration in achieving successful medication delivery. In a study of 300 patients seen in pulmonology clinics, up to 43% of DPI users and 75% of pMDI users displayed incorrect inhaler technique.[7] These errors often go unrecognized, as patients may not demonstrate their inhaler technique to a health care provider.[8] In addition, Restrepro et al.[9] reported that an average of 60% of patients with COPD do not adhere to prescribed therapy. Reasons for nonadherence are multifactorial and include misunderstanding of medications and disease, medication costs, and insufficient health care provider instruction and follow-up.[10] Compromised inhaler technique and medication nonadherence jeopardize health outcomes and add to the economic burden of COPD.[3,10]
The prevalence of COPD rises with age,[11] and the selection of inhaler devices for elderly patients is often complicated by cognitive, physical, reimbursement, and educational challenges. This article discusses special considerations in the selection of inhaler devices for elderly patients with COPD in the outpatient setting and provides recommendations on improving device selection to promote competent inhaler use and medication adherence.
Factors in Device Selection
A previously published systematic review of pertinent randomized controlled trials assessed all methods of aerosol medication delivery, including DPIs, pMDIs, pMDIs with a spacer, and nebulization, among patients with asthma and COPD.[6] After analyzing pooled data from 59 clinical trials, primarily involving use of β2 -adrenergic agonists, the authors concluded that the available evidence showed no significant differences in pulmonary function response with the use of the various delivery devices in the outpatient management of COPD. However, this conclusion was based on data from only seven trials, the majority of which enrolled fewer than 25 patients and did not assess clinical outcomes (e.g., symptom exacerbations or improvement).[6] Instead, the trials analyzed drug delivery to the lungs and clinical response based on controlled laboratory conditions, such as changes in forced expiratory volume (at one second) and dose–response equivalents, and excluded patients who could not use an inhaler device appropriately. Consequently, based on the constraints of available data, the authors recommended that additional factors should be considered when selecting an inhaler device for patients with COPD.[6] These factors include the patient's ability to use the inhaler device properly, the availability of a device with the desired medication, device use with multiple medications, the cost and reimbursement of a device with the desired medication, device convenience (e.g., use in outpatient setting), and patient preference for a device.
Patient's Ability to Use Device
Cognitive Function. Evaluating the patient's ability to use an inhaler device effectively requires the assessment of cognitive capacity for instruction and physical barriers to manipulation of a device. Cognitive impairment is common in patients with COPD and is often related to comorbid conditions such as Alzheimer's disease, Parkinson's disease, and stroke. However, cognitive dysfunction may result from hypoxemia, hypercapnia, or depression that can accompany advancing COPD.[12,13] Cognitive dysfunction in COPD has been evaluated with instruments including the abbreviated mental test score (AMTS) and the Mini-Mental State Examination (MMSE). Cognitive function is an important determinant of the ability to acquire and retain techniques necessary for competent use of inhaler devices. The results of two small trials suggested that patients with significant cognitive dysfunction, as indicated by AMTS scores, could not properly use an MDI.[14,15] However, even patients without overt cognitive impairment (i.e., patients with borderline AMTS or MMSE scores) may be unable to learn techniques for use of both pMDIs and DPIs.[14,16] When self-administration of inhaled medications is being considered, tests of cognitive abilities may be warranted for patients in the advanced stages of COPD and for those of more advanced age.
Dexterity and Strength. Inhaler device manipulation requires manual dexterity and strength, which may be affected by age-related osteoarthritis and neurologic conditions such as Parkinson's disease and stroke.[17] In the advanced stages of these conditions, patients with COPD may need to have trained individuals prepare and administer their nebulized medications. Up to one third of older patients may lack the hand strength to generate the minimum force required to activate a pMDI device.[18,19] Patients with mild-to-moderate impairment of manual dexterity may have difficulty with the hand–breath coordination required to use a pMDI device. Clinicians should order a large-volume spacer to be used with a pMDI device for all elderly patients with mechanical difficulty using pMDIs.[20] DPIs minimize the need for hand–breath coordination, relying instead on breath activation for aerosol generation. However, dosage preparation with single-dose DPI devices may require some level of manual dexterity for loading, puncturing, and disposing of the capsule surrounding each dose.
Medication Availability
Medications for COPD can be categorized as those used in the treatment of acute, worsened symptoms, referred to as "rescue" medications (i.e., short-acting bronchodilators), and those used in managing chronic symptoms, referred to as "controller" medications (i.e., long-acting bronchodilators and inhaled corticosteroids) (Table 1). In the United States, short-acting bronchodilators are available in nebulizer and pMDI formulations but not in DPI formulations. Long-acting bronchodilators and inhaled corticosteroids are available in nebulizer, pMDI, and DPI formulations. Consequently, the use of a DPI for long-term management of symptoms requires the addition of either a pMDI or a nebulizer for intermittent symptom relief. However, use of devices such as the pMDI and DPI in combination may increase the potential for inhaler misuse. Khassawneh et al.[7] compared the inhalation technique of 300 pulmonology clinic patients using pMDIs and DPI devices. The use of more than one type of inhaler increased the likelihood (odds ratio = 2.92) of incorrect handling of either inhaler relative to the use of a single type of inhaler. Patients may misunderstand device-specific instructions, resulting in improper administration technique and severely impaired drug delivery. Choosing the same type of device for treatment of acute and chronic symptoms may facilitate teaching and reduce the potential for confusion, or "device dementia," that may be seen in elderly patients.[6] Alternatively, combined use of a nebulizer and hand-held inhaler may be preferable. Tashkin et al.[21] compared the use of nebulizers only, a pMDI only, or both in combination for administration of albuterol and ipratropium in 126 patients with COPD ranging in age from 62 to 65 years. The authors found that quality-of-life indexes were significantly improved when patients received nebulizer therapy in the morning and at night, with use of a pMDI device for midday doses. They concluded that concomitant use of the devices provided the benefit of additional symptomatic relief offered by a nebulizer and the greater convenience of using a pMDI when away from home.
Cost and Reimbursement
Approximately 96% of the elderly in the United States have health insurance coverage through Medicare.[22] Most outpatient prescription medication coverage is available through Medicare Part D and is provided under a four-stage cycle.[23] On January 1 of each year, the coverage cycle begins with the beneficiary's payment of a deductible (Stage I); that is followed by a cost-sharing arrangement (Stage II) involving a limited patient co-payment, with provider payment of medication expenses up to $2830 annually. If annual patient and provider expenses exceed $2830, a coverage gap (Stage III) develops, and the patient must pay 100% of medication costs up to $4550, at which point (Stage IV) a patient copayment is reinstated and remains in effect until the end of the year, when the cycle begins anew.
At any point in time, elderly patients with COPD may be receiving an average of 6.2 prescription medications, often for comorbid or age-related illnesses.[9] As a result, the patient cost of combination controller medications, such as fluticasone propionate–salmeterol xinafoate (Advair, GlaxoSmithKline, Research Triangle Park, NC) or budesonide–formoterol fumarate (Symbicort, AstraZeneca, Wilmington, DE), must be considered, as use of these products may lead to rapid development of a coverage gap. Annual patient and provider expenses associated with these controller medications can nearly exhaust a beneficiary's Stage II Medicare Part D coverage (e.g., the annual cost for combination fluticasone propionate 250 μg–salmeterol xinafoate 50 μg twice daily is an estimated $2500).[24] In that event, the patient will have a limited allowance for any additional medication expenses and might incur substantial out-of-pocket costs for the rest of the year. In 2010, a new health care reform law promised to close the coverage gap by the year 2020, with a 50% discount on branded prescription drugs in 2011.[25] While these efforts will improve medication expenses, the overall impact on cost savings will be determined by medication prices set by the pharmaceutical industry.
To receive Medicare Part D prescription drug coverage, patients must also subscribe to Medicare Parts A and B. Part B medication coverage is limited to drugs administered through durable medical equipment, including nebulizers and compressors, and medications that are available as inhalation solutions. Patient expenses under Medicare Part B include a premium, a deductible, and a copayment (unlike Part D benefits, there is no coverage-gap rule). Therefore, nebulized formulations offer significant cost advantages to the patient, by increasing allowances in Stage II of the coverage cycle and reducing out-of-pocket (Stage III) expenses among Medicare Part D beneficiaries.[26] These cost and reimbursement factors may affect patient adherence to therapy and thus warrant early consideration in elderly patients with COPD.
Device Considerations and Patient Preferences
MDIs
The pMDI has been called the most complex dosage form in medicine.[27] Conceived by Riker Laboratories in 1955, the pMDI was developed to improve the ease of use of aerosolized medications previously delivered through nebulizers.[6] The most distinct advantages of a pMDI over a nebulizer are conveniences, including multiple dosing (≥100 doses/canister), shorter administration time, and the compactness and portability of the device. However, the use of a pMDI requires a specific inhalation technique that depends on adequate coordination to generate a respirable dose (Table 2).[27–30]
The pMDI device consists of a canister, a metering chamber, and an actuator with a mouthpiece. Medication is found in solution with a liquefied gas propellant (hydrofluoroalkane [HFA]) and is housed in a pressured canister fitted with a metering valve. A plastic actuator with a molded mouthpiece and actuator nozzle holds the canister in position for medication delivery. The pressure needed for aerosolization of the medication is created in the canister by shaking the device to disperse the liquefied gas propellant into the medication solution. As the canister is depressed into the plastic actuator, one metered dose of medication is delivered in a high-velocity spray via the actuator nozzle to the mouthpiece (Figure 1).[27,29] The high-velocity aerosol plume only lasts 100–400 milliseconds as the size of particles continues to diminish due to the evaporation of the HFA propellant.[29,30] Aerosolized particles may diminish in size from 45 μm at the actuator nozzle to 0.5 μm 6 inches from the mouthpiece.[30] Particle size is a key determinant of the respirable dose. Larger particles (>10 μm) are highly likely to remain in the oropharynx; midsized particles (5–10 μm) remain in the upper large bronchial tree, while those of optimum size (1–5 μm) reach the lower airways. Smaller particles (≤0.5 μm) are not deposited in the lungs but are instead exhaled as a gas.[30,33] However, even with correct inhaler technique, most pMDIs only deposit 10–20% of the labeled dose in the lungs; much of the medication remains in the mouth and oropharynx, potentially leading to local and systemic adverse effects.[5,29,30]
Figure 1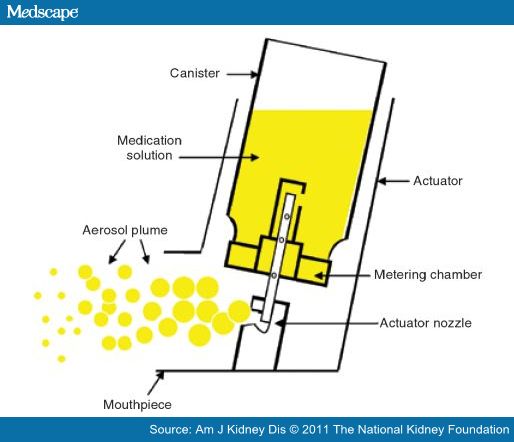
Components of a pressurized metered-dose inhaler. Adapted, with permission, from Fromer L, Goodwin E, Walsh J. Customizing inhaled therapy to meet the needs of COPD patients. Postgrad Med. 2010; 122:83–93.
Due to the limited medication deposition and narrow range of respirable particle sizes seen with use of pMDI devices, appropriate patient inhalation technique is necessary for optimum medication delivery. There are multiple steps required for proper technique. Essential yet frequently omitted steps in the proper use of a pMDI are (1) shaking of the device, which disperses propellant for aerosolization, (2) simultaneous actuation of the device and synchronous slow inhalation to allow medication particles of a respirable size to develop, and (3) holding the breath for at least six seconds after the inhalation to allow deposition of respirable particles in the airways.[5,33] During an observational trial involving a total of 3811 patients, at least one of those critical steps was omitted in 76% of patient demonstrations.[34] McFadden[30] characterized patient errors with pMDIs based on studies of nearly 1000 patients. The most commonly observed error, seen in 27% of patients, was the failure to synchronize inhalation with pMDI actuation (i.e., poor "hand–breath coordination"), which leads to lower respirable doses and medication delivery.[5,28,30] In 26% of patients, inadequate breath holding occurred, causing optimally sized particles to be exhaled instead of deposited in the lower airways.[5,30] Another critical error was overly rapid inspiration (seen in 19% of patients),[30] which leads to oropharyngeal deposition instead of pulmonary delivery due to inhalation of larger particles.[28,32]
Use of spacer devices helps overcome some of the more common problems with inhalation technique, and spacers have been recommended for all elderly patients using a pMDI device.[21,35] The purpose of a spacer is to slow the aerosol spray before it reaches the mouth; that allows the propellant to evaporate, reducing particles to a respirable size and holding the medication dose until it is inhaled by the patient.[30] Most importantly, spacers eliminate the need to coordinate inhalation with actuation, the most common error seen with pMDI use.[28] Spacers also reduce oropharyngeal deposition of medication by allowing particle size reduction in the tube or holding chamber.[35] Conveniently, many spacer devices emit a signal (such as a vibrating reed) warning the user that inspiration is occurring too quickly.[32] However, spacers are bulky and may increase the time required for medication administration due to the need for device assembly.[5] In addition, the walls of the holding chamber of most spacers carry an electrostatic charge that attracts drug particles, thus reducing the respirable dose.[5,28,32] This problem (and the risk of infection) can be minimized through weekly washing of the spacer alongside air drying, but such measures add maintenance time and inconvenience to use of the pMDI.[35]
The technical challenges posed by pMDIs and the inconveniences of using a spacer may significantly contribute to patient preference for other inhaler devices. In a study of inhalation technique and device preferences, 100 patients with asthma or COPD were instructed on the use of seven different pMDI and DPI devices; they were then evaluated for inhalation technique and asked to choose three preferred devices. A higher proportion of patients exhibited proper technique with the DPIs compared with the pMDIs (91% versus 79%), and they expressed a strong preference for the DPIs, although 55% indicated current use of a pMDI.[36] In a multicenter, randomized, open-label crossover study, Welch et al.[37] compared patient preference for budesonide inhalation powder (Pulmicort Turbuhaler, AstraZeneca) via a DPI device versus one of three other inhaled corticosteroids delivered via a pMDI; patients rated the DPI device significantly higher for ease of use (p = 0.0005) and overall satisfaction (p < 0.0001), despite previous use of pMDIs by most patients in the study. The findings in both studies indicated that patients are more likely to use the inhaler device they prefer.[36,37] Therefore, patient preference should be strongly considered in device selection.[6]
DPIs
The DPI is a breath-actuated device developed to overcome the difficulty of achieving the proper hand–breath coordination necessary to actuate a pMDI device for effective drug delivery.[6] Like pMDIs, DPIs have advantages over nebulizers, including the convenience of a compact, portable device and rapid medication delivery. Unlike pMDIs, many DPIs (i.e., multidose devices) have dose counters, which indicate the amount of medication remaining. In addition, some DPI devices emit inspiratory-flow signals that promote proper technique and patient adherence (Table 2).[6,38]
A DPI device consists of a medication reservoir, air inlet, deagglomeration compartment, and mouthpiece.[39] Medication formulations for DPIs are micronized drug particles either in a pure form or bound to an inert, larger carrier molecule (such as lactose) to form loose agglomerates. Rapid patient inspiration passes the drug formulation through a screen of spinning surfaces or generates turbulent airflow that deaggregates drug particles into a respirable dose (Figure 2).[5,32,38] Accordingly, use of DPIs requires inspiratory abilities sufficient to deaggregate the medication formulation into particles suitable for lung deposition.[32] Delivery of medication is dependent on the peak inspiratory flow rate (PIFR) that a patient is able to generate through the device;[38,39] this threshold peak inspiratory flow is, in turn, dependent on the internal resistance of the inhaler and is thus device specific.[40] A patient-generated PIFR of greater than 60 L/min is considered ideal for use of most DPI devices.[41] Conversely, a PIFR of less than 30 L/min may be insufficient for optimal pulmonary deposition, while rates of 30–60 L/min may still provide sufficient therapeutic effects.[41] Among DPI devices available in the United States, only the Spiriva Handihaler (Boehringer-Ingelheim, Ridgefield, CT) requires a PIFR of at least 30 L/min for delivery of an optimal respiratory dose.[42] In an observational study of the use of four DPIs among 224 patients with COPD, the rate of ineffective inhalation due to inadequate peak expiratory flow was correlated with patient age and the severity of airway obstruction.[43] Elderly patients with severe COPD may not achieve a sufficient PIFR, which may compromise the delivery of the intended dose.[44] With a growing number of COPD patients who are over 70 years old and have advanced disease, the convenience of a DPI must be weighed against the individual patient's ability to generate the required device-specific peak inspiratory flow.[45] In such cases, a metered device that mimics the internal resistance of various DPIs and measures a patient's inhalation rate (e.g., In-Check Dial, Alliance Tech Medical, Granbury, TX) can help determine the suitability of a specific DPI for a particular patient.[45] If such testing cannot be performed, inadequate inspiratory flow should be suspected in patients with very severe COPD or poor respiratory effort.[46]
Figure 2
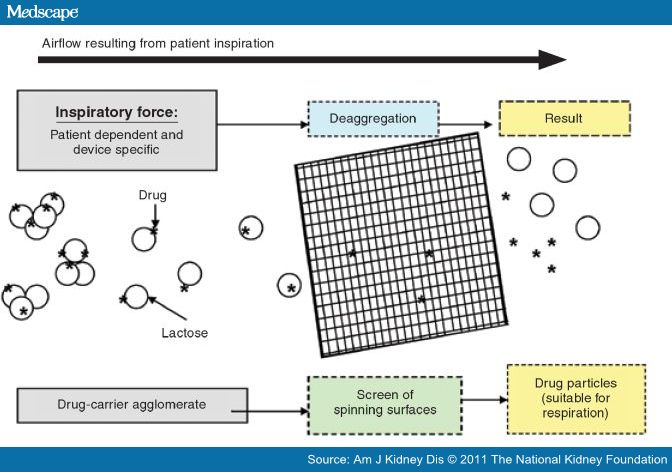
Mechanism of a dry powder inhaler. The drug is weakly bound to a carrier (e.g., lactose) in powder form. The force resulting from the patient's inspiration causes deaggregation of the drug from the carrier and disperses particles into a fine respirable fraction. Adapted from reference 45, with permission. Copyright © 2003 Elsevier.
Humidity can also have a large effect on drug delivery through a DPI due to the potential for clumping of drug powder. Meakin et al.[47] found that the nominal dose per use listed in the labeling of one multidose reservoir device (Turbuhaler) was reduced by 20–55% with exposure to ambient relative humidity of 70% for two hours; this effect was found to persist for up to four days. A much smaller reduction in the dose delivered under similar conditions was seen with a device with individually sealed unit doses of drug (Diskus, GlaxoSmithKline) because the drug powder is contained within a protective blister strip that is not pierced until the dose is administered.[48] Both of these studies highlighted the importance of storing DPI devices in a cool, dry environment and avoiding their use in the bathroom or outdoors on a humid day.[5,49]
Available DPIs include single- and multiple-dose devices. With single-dose products (e.g., Aerolizer, Novartis, Basle, Switzerland; Handihaler, Boehringer-Ingelheim), the DPI must be loaded before each inhalation, usually with a capsule containing powder.[39] Once loaded, the capsule is pierced within the device and the powder is inhaled. The spent capsule must be removed, and a new capsule inserted for the next dose.[49] Loading, puncturing, and discarding the capsule require some manual dexterity and strength, which may pose a problem for the elderly and patients with severe shortness of breath.[49] Also, there have been reports of patients intentionally swallowing the capsules instead of inhaling the contents.[27] In addition, patients may need to take two or more breaths to inhale the therapeutic dose from the device due to high internal resistance.[39,49] As a precaution, patients should be instructed to check the capsule in the device after the first inhalation and repeat the inhalation process if the capsule is not empty. The labeling of one single-dose device for the delivery of formoterol fumarate (Foradil, Novartis) stipulates refrigeration of the medication capsules prior to dispensing. The need for such additional steps and instructional measures may contribute to an already cumbersome process and lead to poor acceptance by patients.[49]
Multiple-dose DPI devices either deliver an individual dose of powder from a reservoir (e.g., Turbuhaler; Twisthaler, Schering, Kenilworth, NJ) or deliver premeasured individual doses from blisters, disks, or strips (e.g., Diskhaler, GlaxoSmithKline).[39] Patients tend to favor multiple-dose devices due to their ease and quickness of use, generally lower costs, and integral dose counters that allow patients to view the level of remaining medication.[28,49]
Nebulizers
Of all the aerosolized drug delivery systems, nebulizers are the easiest for patients to use,[5] requiring minimal cognitive abilities and virtually no hand–breath coordination, manual dexterity, or hand strength.[50] The mist generated by nebulizers may foster confidence in patients, providing visible evidence that they are receiving the medication.[28] Balzano et al.[51] performed a two-week crossover study in elderly patients with COPD or asthma who received respiratory medications through a jet nebulizer and a pMDI device. After using both delivery devices, patients expressed a clear preference for the nebulizer with regard to effectiveness, but they preferred the pMDI with regard to acceptability. In a study by Barta et al.,[52] which surveyed 75 patients receiving outpatient nebulizer therapy for chronic lung disease, 56–91% of patients reported that nebulizer use conferred improved symptom control, well-being, and self-confidence despite the relatively long duration of administration and device reliability and technical issues.
The two main types of nebulizer for aerosol drug delivery are the compressor-driven jet nebulizer and the ultrasonic nebulizer.[53] Ultrasonic nebulizers consist of an electric power unit, a transducer, and a fan. Medication is placed directly over the transducer or in a medication chamber. The power unit vibrates the transducer, creating high-frequency vibrations that produce ultrasonic waves, which pass through the medication solution.[54] The ultrasonic waves aerosolize the solution while a fan evacuates the aerosol from the device for delivery to the patient. Because they are not driven by a compressor, ultrasonic nebulizers are quieter than jet nebulizers.[54] In addition, small-volume ultrasonic nebulizers require less medication solution than small-volume jet nebulizers, allowing faster medication delivery. Battery-powered, rapid- delivery, compressor-free small-volume ultrasonic nebulizers provide portability and convenience to patients. However, while ultrasonic nebulizers have been promoted for administration of bronchodilators as well as antiinflammatory agents, the machines typically do not readily aerosolize drug suspensions.[28] The manufacturer of budesonide, a respiratory suspension, recommends against its use with an ultrasonic nebulizer due to potential inadequate dose delivery.[55] In contrast, jet nebulizers are recommended for delivery of all medication solutions and suspensions intended for nebulization in COPD.[53,54]
The jet nebulizer consists of a medication reservoir linked by tubing to a battery- or ac-powered compressor, which forces pressurized gas through the tubing and into contact with the medication reservoir. The liquid medication is mixed with the compressed gas to form droplets; a stream of droplets rises in the nebulizer to a baffle that aerosolizes the droplets for inhalation (Figure 3).[53,54]
Figure 3
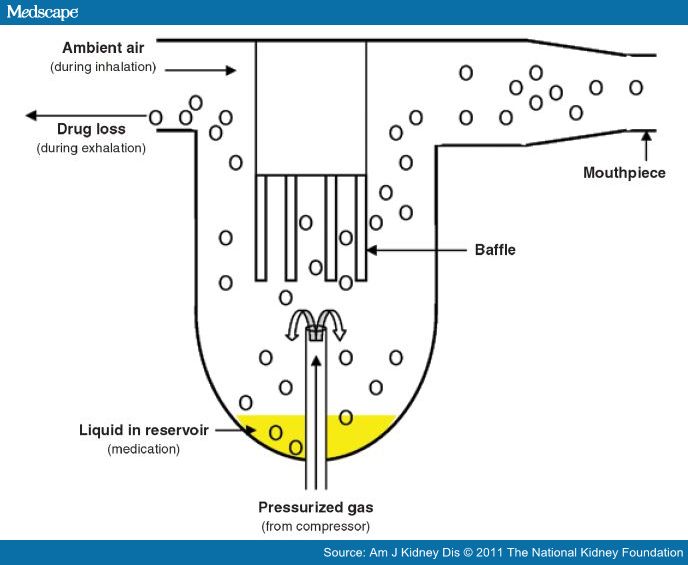
Basic components of a nebulizer. Adapted, with permission, from Newman SP. Aerosol generators and delivery systems. Respir Care. 1991; 36:939–51.
The disadvantages of both jet and ultrasonic nebulizers include the long duration of administration, poor portability, the need for daily cleaning, and the trouble and cost associated with proper maintenance and replacement of the nebulizers and compression units. It takes considerably longer to deliver a complete respirable dose with a nebulizer than with a pMDI or DPI.[28] Typically, a small-volume jet nebulizer can be expected to deliver more than 50% of the total dose at an appropriate particle size in 10 minutes or less.[54] Appropriate, frequent cleaning—ideally after each use—is a very important aspect of the care of the nebulizer, whether it is a disposable or a reusable unit. Cleaning usually includes disassembling the nebulizer, soaking all components in warm soapy water, rinsing, and air drying before the next use. When multiple drugs and frequent treatments are required, patients can have more than one nebulizer available for immediate use.[56] Cleaning measures help extend the durability of the nebulizer. One study found that nebulizers that were not cleaned after each use began to fail after only 40 uses.[57] With proper cleaning, reusable nebulizers generally can provide safe and effective drug delivery for six months; disposable nebulizers are typically replaced after just two weeks. For optimal durability, manufacturer recommendations for cleaning should be followed. Routine cleaning following each treatment, with disinfecting after every second treatment day, can help prevent infection or reinfection in a population of patients at increased risk for immune system compromise.[58] Patients with minimal cognitive abilities or restricted hand strength and dexterity require a trained provider to prepare and administer medication and clean the device.
Education Challenges
Suboptimal inhaler technique is a common problem among patients with respiratory disease. The use of inhaler devices requires multiple steps to ensure effective medication delivery. In an observational study of 3811 patients with COPD or asthma, critical errors in technique that could significantly compromise medication delivery were observed in 28% of pMDI users and, depending on the specific device, 11–32% of DPI users, emphasizing the need for ongoing educational efforts.[34] It has been said that management of chronic airway disease is "10% medication and 90% education."[40]
Almost 25% of the adult population of the United States cannot read and understand basic written instructions.[40] Not surprising, in a study of patients who had not previously used inhaler devices, only 21% of patients were able to use a pMDI correctly after reading the package insert.[59] To learn appropriate inhaler technique, written instructions alone are insufficient. One published review of randomized trials indicated that teaching interventions more than doubled the likelihood of correct inhaler use.[60] Yet, up to 25% of patients may receive no oral instruction on inhaler use.[50] In addition, patients who learn to use their medication delivery devices require repeated assessment and instruction to reinforce correct technique.[61] A Canadian audit found that 28% of patients had never demonstrated their inhaler technique to a health care provider.[8] Unfortunately, studies have shown that many health care providers themselves have limited knowledge of correct inhaler technique. A review of 20 studies suggested that the majority of health care providers, including nursing home staff, may lack competence in the use of pMDI, spacers, and DPI devices.[62]
With their relative accessibility and medication expertise, pharmacists play a vital role in the instruction of patients on inhalation technique and in the assessment of inhaler therapy effectiveness and adherence. In a survey of community pharmacists, at least 85% of respondents reported counseling patients on new prescriptions of inhaler devices; however, the proportion reporting sustained counseling efforts dropped to less than 69% within three months and to 29% beyond three months.[63] Like other groups of health care professionals, pharmacists often lack knowledge of the skills necessary for appropriate use of inhaler devices.[63] Studies of community pharmacies have indicated rates of pharmacist competency in performing all steps of inhaler administration of only 29–72%.[62] This deficiency can lead to diminished patient confidence and understanding, hindering patient counseling efforts while potentially leading to incorrect device use and worsened control of COPD. To help ensure a sound knowledge base, pharmacists should use available educational resources for devices commonly used by their patients. Pharmacist counseling involving visual information and device demonstration is effective in improving inhalation technique and patient respiratory symptoms.[64,65] A checklist of inhalation technique may prove helpful for pharmacist and patient understanding of medication administration (appendix).[29,42,55,66–68] Pharmacists should regularly evaluate and educate their patients on the use of inhaler devices to help ensure patient adherence throughout the course of therapy. In the community setting, markers for inhaler underuse and incorrect use may include missed refills of controller medications and more frequent refills of rescue medications. In the hospital setting, inhaler technique should be assessed during every visit to the emergency room or admission for respiratory distress.[69] Finally, the pharmacist may play an integral role in educating other educators through their affiliations and correspondence with health care providers, including those in skilled nursing facilities and clinics.[70]
Discussion
The purpose of selecting an appropriate inhaler device is to improve patient adherence and inhaler technique to achieve the goals of therapy for patients with COPD. In 2005, the American College of Chest Physicians and the American College of Asthma, Allergy, and Immunology jointly issued evidence-based guidelines on key considerations in the selection of inhaler devices for patients with asthma or COPD.[6] Since that time, the introduction of new products and formulations and the advent of Medicare Part D have further complicated device selection. In elderly patients with sufficient cognitive function, manual dexterity, and hand strength, the most important factors in inhaler selection are cost and reimbursement concerns, device availability, convenience issues, and patient preference.
In many cases, cost and reimbursement concerns may be a deciding factor in device selection. Nearly all elderly patients are Medicare beneficiaries. Medications administered via pMDI and DPI devices require Medicare Part D. Routine administration of a pMDI or DPI formulation of a combination of controller medications (i.e., a long-acting bronchodilator and an inhaled corticosteroid) will promote patient expenses during the coverage gap; this is especially relevant to patients receiving multiple prescription medications not available in generic formulations. However, all patients with Medicare Part D also have Part B coverage, which includes coverage of about 80% of the cost of nebulizer equipment and medication expenses (a potentially key factor in averting a coverage-gap situation); thus, nebulizer therapy may be more affordable than the use of pMDI and DPI devices for many elderly patients with COPD.
Medication availability issues and inhaler device convenience features can be key factors in whether clinician and patient preferences can be accommodated. Some preferred medications are not available in pMDI or DPI formulations. Both rescue and controller medications are available in formulations for nebulizer administration. The portability of battery-powered small-volume nebulizers has improved, and many patients feel reassured of medication administration by the visible mist; however, relatively complex device preparation and maintenance requirements, as well as the long duration of administration, continue to limit the use of these nebulizers. Patients' preference for pMDIs and DPIs is most notable for easy handling and quick multiple-dose administration. While DPIs often have convenient dosing counters and offer unprecedented ease of use, current devices are only available in controller-medication formulations. The use of pMDIs requires hand–breath coordination that many elderly patients cannot achieve; spacer devices help to overcome that problem, but their use entails reduced device portability and additional time for maintenance and assembly. In light of the availability and expenses of medication devices, ambulatory patients may prefer to use two types of inhaler devices concurrently, thereby combining the convenience of a hand-held inhaler (i.e., pMDI) as a rescue medication during the active hours of midday with the cost savings of a controller medication administered through a nebulizer in the morning and at night.
Regardless of the device selected, patient education is instrumental in optimizing inhaler technique and medication adherence. Oral instructions and demonstrations, with ongoing assessment and reinstruction, are essential for effective education of patients on use of inhaler devices. Pharmacists are well qualified to provide these educational services and often more accessible than other health care providers; however, evidence suggests that many pharmacists may lack competence in correct inhaler technique, potentially resulting in suboptimal inhaler therapy and, ultimately, worse COPD treatment outcomes. Wider recognition and remediation of many pharmacists' poor knowledge of inhaler technique are necessary. Confident, effective instruction will help ensure proficient use of inhaler devices and long-term patient adherence to therapy. Regular follow-up assessment and reinstruction reinforce patient competency and attainment of medication goals while helping clinicians identify barriers to adherence that may necessitate the consideration of alternative inhaler devices.
Conclusion
In elderly patients with sufficient cognitive function, manual dexterity, and hand strength, the most important factors in inhaler device selection are cost reimbursement issues, device availability, device convenience, and patient preference. Pharmacist knowledge of appropriate inhaler technique, competent patient education and demonstration, and follow-up assessment are instrumental in optimizing device competency and medication adherence.
Appendix Patient Counseling Checklists29,42,55,66,68
Pressurized Metered-dose Inhaler
- Remove mouthpiece cap
- Shake inhaler
- Hold inhaler upright (sit or stand straight)
- Breathe out (completely)
- Put mouthpiece between lips (seal lips tightly around)
- Take in a slow deep breath while depressing the canister
- Hold breath for at least 10 seconds
- Wash device (warm water only) at least once weekly and air dry
Special considerations:
• If second inhalation is required, wait at least 30 seconds and then repeat steps beginning at "shake inhaler."
• If medication is an inhaled corticosteroid, swish mouth with water after each dose and spit contents.
Dry Powder Inhaler
- Remove cover and/or open device (varies with device)
- Load device (only pertains to capsule for single-dose device)
- Hold device upright; do not shake
- Prime device (varies with device; see special considerations)
- Breathe out completely but do not exhale into device
- Place mouthpiece between lips (seal lips tightly around)
- Take a quick deep breath (repeat until capsule is empty for single-dose device)
- Hold breath for at least 10 seconds
- Maintain device per specific instructions (see special considerations)
Special considerations:
•If medication is an inhaled corticosteroid, swish mouth with water after each dose and spit contents.
•Prime single-dose device (e.g., Foradil Aerolizer, Spiriva Handihaler) by piercing capsule.
• Prime multiple-dose device by listening for clicking sound after either sliding lever (e.g., Advair, Serevent Diskus) or twisting base (e.g., Pulmicort Flexhaler, Asmanex Twisthaler).
• Wash single-dose dry powder inhaler device (in warm water) once weekly and air dry.
• Do not expose multiple-dose dry powder inhaler devices to water.





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 