
Severe acute alcoholic hepatitis is a life-threatening alcoholic liver disease.1 Although glucocorticoid treatment is recommended2,3 and improves survival,4-11 mortality remains high, with 35% of patients dying within 6 months.1Long-term alcohol consumption increases intestinal permeability, worsens endotoxemia,12 stimulates Kupffer cells,13 and thus increases production of proinflammatory cytokines.14 High levels of tumor necrosis factor α (TNF-α) activate cell-death pathways and induce the production of reactive oxygen species, notably superoxide anions, by the hepatocyte mitochondria, leading to cell death. This situation is accompanied by severe mitochondrial depletion of glutathione,15 the primary antioxidant in cells. Furthermore, hepatocytes are much more sensitive to TNF-α when their antioxidant reserves are low. 16 Combination therapy with an antioxidant and glucocorticoids would have the advantage of both acting on the inflammatory process and reconstituting cellular glutathione reserves.N-acetylcysteine could have value as an antioxidant in the treatment of acute alcoholic hepatitis, because the thiol group in N-acetylcysteine is able to reduce levels of free radicals. Administration of N-acetylcysteine might reconstitute the glutathione stocks of the hepatocytes. At present, N-acetylcysteine is used in the treatment of acetaminophen-induced hepatitis. We conducted a trial to evaluate the efficacy of glucocorticoids plus N-acetylcysteine, as compared with glucocorticoids alone, in patients with severe acute alcoholic hepatitis.
Severe acute alcoholic hepatitis is a life-threatening alcoholic liver disease.1 Although glucocorticoid treatment is recommended2,3 and improves survival,4-11 mortality remains high, with 35% of patients dying within 6 months.1Long-term alcohol consumption increases intestinal permeability, worsens endotoxemia,12 stimulates Kupffer cells,13 and thus increases production of proinflammatory cytokines. High levels of tumor necrosis factor α (TNF-α) activate cell-death pathways and induce the production of reactive oxygen species, notably superoxide anions, by the hepatocyte mitochondria, leading to cell death. This situation is accompanied by severe mitochondrial depletion of glutathione, the primary antioxidant in cells. Furthermore, hepatocytes are much more sensitive to TNF-α when their antioxidant reserves are low. 16 Combination therapy with an antioxidant and glucocorticoids would have the advantage of both acting on the inflammatory process and reconstituting cellular glutathione reserves.N-acetylcysteine could have value as an antioxidant in the treatment of acute alcoholic hepatitis, because the thiol group in N-acetylcysteine is able to reduce levels of free radicals. Administration of N-acetylcysteine might reconstitute the glutathione stocks of the hepatocytes. At present, N-acetylcysteine is used in the treatment of acetaminophen-induced hepatitis. We conducted a trial to evaluate the efficacy of glucocorticoids plus N-acetylcysteine, as compared with glucocorticoids alone, in patients with severe acute alcoholic hepatitis.
Study Treatments
Both groups received 40 mg of oral prednisolone per day for 28 days. For the first 5 days, patients in the prednisolone–N-acetylcysteine group received intravenous infusions of N-acetylcysteine (Fluimucil, Zambon Group). On day 1, they received 150 mg per kilogram of body weight in 250 ml of 5% glucose solution over a period of 30 minutes, 50 mg per kilogram in 500 ml of glucose solution over a period of 4 hours, and 100 mg per kilogram in 1000 ml of glucose solution over a period of 16 hours. On days 2 through 5, they received 100 mg per kilogram per day in 1000 ml of glucose solution. The patients in the prednisolone-only group received an infusion in 1000 ml of 5% glucose solution per day on days 1 through 5.The treatment of ascites with diuretics, albumin, and sodium restriction was allowed, as was the use of beta-blockers for portal hypertension. Management of alcohol addiction was left to the individual center. The use of acetaminophen, pentoxifylline, or anti–TNF-α treatments was prohibited. All patients received normal hospital nutrition (1800 to 2000 kcal per day).Study OutcomesThe primary outcome was survival at 6 months. Prognostic factors for 6-month mortality were examined. The secondary outcomes were survival at 1 and 3 months, changes in bilirubin levels after 7 and 14 days of treatment, occurrence of hepatitis complications, and adverse events related to N-acetylcysteine use. Liver transplantation or use of the molecular adsorbent recirculating system (MARS) during the trial was treated as a mortality end point in survival analyses.Study OversightThe study was approved by the institutional review board at Amiens University Hospital and was conducted in compliance with the French Huriet–Sérusclat legislation on medical research and the Declaration of Helsinki. All patients provided written informed consent before enrollment. (For patients with hepatic encephalopathy, informed consent was obtained from the next of kin.)Statistical AnalysisPrimary and secondary outcomes were compared between the two treatment groups. Quantitative variables, expressed as means ±SD, were compared with the use of the Wilcoxon test, Kruskal–Wallis test, or Student's t-test, as appropriate. Qualitative variables, expressed as percentages, were compared with the use of a chi-square test or Fisher's exact test. Kaplan–Meier survival curves were plotted for up to 180 days and compared with the use of a log-rank test. Factors that were significantly predictive of mortality in a univariate analysis (P<0.05) were included in a multivariate Cox logistic-regression analysis with stepwise elimination. A bilirubin decrease on day 7 or 14 was defined as a lower absolute value than on day 0. In secondary analyses that were not prespecified in the study protocol, we compared causes of death between the two groups and examined the effect of change in bilirubin levels over time on survival. An intermediate safety analysis was performed after 50% of the enrolled patients had completed 6 months of follow-up (with P<0.05 chosen as the threshold for statistical significance). For the final analysis, a P value of less than 0.025 was used. All the statistical analyses were performed in the modified intention-to-treat population. All reported P values are two-sided.The required sample size was calculated on the assumption that the survival rate would be 67% at 6 months in the prednisolone-only group.1 With an alpha error of 0.05, a beta error of 0.10, and a hypothetical improvement in survival of 20% at month 6 for the prednisolone–N-acetylcysteine group, the required sample size was 174 patients.
Study TreatmentsBoth groups received 40 mg of oral prednisolone per day for 28 days. For the first 5 days, patients in the prednisolone–N-acetylcysteine group received intravenous infusions of N-acetylcysteine (Fluimucil, Zambon Group). On day 1, they received 150 mg per kilogram of body weight in 250 ml of 5% glucose solution over a period of 30 minutes, 50 mg per kilogram in 500 ml of glucose solution over a period of 4 hours, and 100 mg per kilogram in 1000 ml of glucose solution over a period of 16 hours. On days 2 through 5, they received 100 mg per kilogram per day in 1000 ml of glucose solution. The patients in the prednisolone-only group received an infusion in 1000 ml of 5% glucose solution per day on days 1 through 5.The treatment of ascites with diuretics, albumin, and sodium restriction was allowed, as was the use of beta-blockers for portal hypertension. Management of alcohol addiction was left to the individual center. The use of acetaminophen, pentoxifylline, or anti–TNF-α treatments was prohibited. All patients received normal hospital nutrition (1800 to 2000 kcal per day).
Study Outcomes
The primary outcome was survival at 6 months. Prognostic factors for 6-month mortality were examined. The secondary outcomes were survival at 1 and 3 months, changes in bilirubin levels after 7 and 14 days of treatment, occurrence of hepatitis complications, and adverse events related to N-acetylcysteine use. Liver transplantation or use of the molecular adsorbent recirculating system (MARS) during the trial was treated as a mortality end point in survival analyses.
Study Oversight
The study was approved by the institutional review board at Amiens University Hospital and was conducted in compliance with the French Huriet–Sérusclat legislation on medical research and the Declaration of Helsinki. All patients provided written informed consent before enrollment. (For patients with hepatic encephalopathy, informed consent was obtained from the next of kin.)
Statistical Analysis
Primary and secondary outcomes were compared between the two treatment groups. Quantitative variables, expressed as means ±SD, were compared with the use of the Wilcoxon test, Kruskal–Wallis test, or Student's t-test, as appropriate. Qualitative variables, expressed as percentages, were compared with the use of a chi-square test or Fisher's exact test. Kaplan–Meier survival curves were plotted for up to 180 days and compared with the use of a log-rank test. Factors that were significantly predictive of mortality in a univariate analysis (P<0.05) were included in a multivariate Cox logistic-regression analysis with stepwise elimination. A bilirubin decrease on day 7 or 14 was defined as a lower absolute value than on day 0. In secondary analyses that were not prespecified in the study protocol, we compared causes of death between the two groups and examined the effect of change in bilirubin levels over time on survival. An intermediate safety analysis was performed after 50% of the enrolled patients had completed 6 months of follow-up (with P<0.05 chosen as the threshold for statistical significance). For the final analysis, a P value of less than 0.025 was used. All the statistical analyses were performed in the modified intention-to-treat population. All reported P values are two-sided.The required sample size was calculated on the assumption that the survival rate would be 67% at 6 months in the prednisolone-only group.1 With an alpha error of 0.05, a beta error of 0.10, and a hypothetical improvement in survival of 20% at month 6 for the prednisolone–N-acetylcysteine group, the required sample size was 174 patients.
RESULTS
Study Population A total of 430 patients were evaluated for eligibility (Figure 1 Enrollment and Outcomes. ).
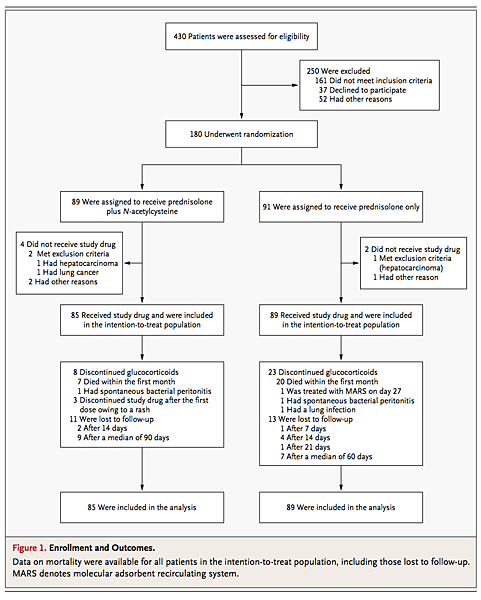
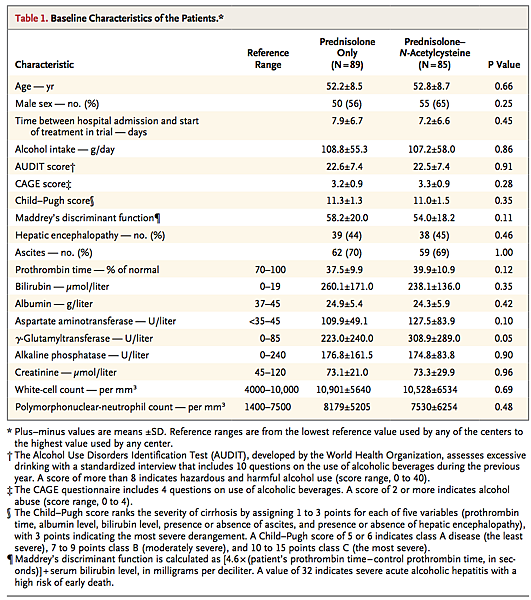
After exclusion of 250 patients who did not meet the inclusion criteria or for other reasons, 180 underwent randomization. The final analysis was performed with 174 patients (73 from Amiens, 39 from Besançon, 20 from Caen, 15 from Saint-Quentin, 11 from Rouen, 7 from Cambrai, 2 from Saint-Antoine, 2 from Pitié-Salpêtrière, 2 from Abbeville, 2 from Beauvais, and 1 from Reims). The baseline characteristics of the patients did not differ significantly between the two groups, with the exception of a slightly higher γ-glutamyltransferase level in the prednisolone–N-acetylcysteine group (P=0.05) (Table 1 Baseline Characteristics of the Patients. ).
The results of the scheduled interim analysis have been published previously.
Mortality
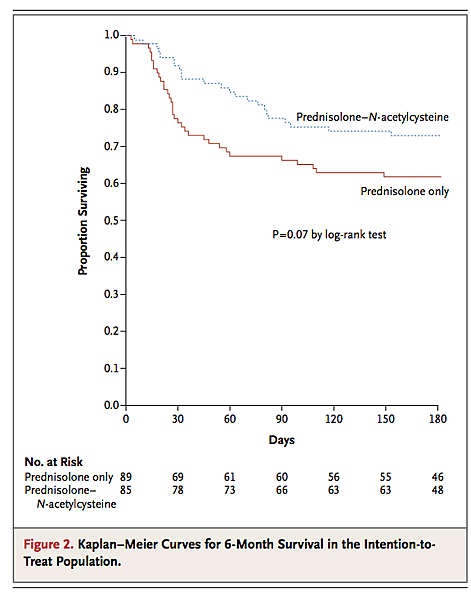
In regard to the primary outcome, 57 patients had died by 6 months. The mortality rate was 38% in the prednisolone-only group (34 of 89) and 27% in the prednisolone–N-acetylcysteine group (23 of 85) (hazard ratio with combination therapy, 0.62; 95% confidence interval [CI], 0.37 to 1.06; P=0.07) (Figure 2
Kaplan–Meier Curves for 6-Month Survival in the Intention-to-Treat Population.).
The mean time to death was 40±35 days (median, 27; range, 3 to 149) in the prednisolone-only group and 54±37 days (median, 50.5; range, 5 to 149) in the prednisolone–N-acetylcysteine group (P=0.14). In regard to the secondary outcomes, the respective mortality rates in the prednisolone-only and prednisolone–N-acetylcysteine groups were 24% (21 of 89) and 8% (7 of 85) at 1 month (hazard ratio, 0.58; 95% CI, 0.14 to 0.76; P=0.006) and 34% (30 of 89) and 22% (19 of 85) at 3 months (hazard ratio, 0.33; 95% CI, 0.33 to 1.04; P=0.06).
Causes of Death
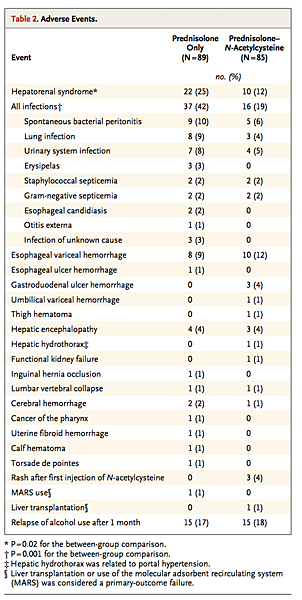
At 6 months, 22% of the patients in the prednisolone-only group (20 of 89) had died of the hepatorenal syndrome, versus 9% of the patients in the prednisolone–N-acetylcysteine group (8 of 85) (odds ratio, 2.79; 95% CI, 1.08 to 7.42; P=0.02); the mean time to death was 36.5±28 days and 66±33 days, respectively (P=0.30). Deaths due to infections accounted for 9% of patients in the prednisolone-only group (8 of 89) and 8% of those in the prednisolone–N-acetylcysteine group (7 of 85) (odds ratio, 0.91; 95% CI, 0.28 to 2.93; P=0.85), with a mean time to death of 31±22 days and 56±45 days, respectively (P=0.05). In the prednisolone-only group, 4 patients died from septic shock, 3 from lung infections, and 1 from spontaneous bacterial peritonitis; in the prednisolone–N-acetylcysteine group, 2 patients died from septic shock, 2 from lung infections, and 1 each from subphrenic abscess, spontaneous bacterial peritonitis, and pyelonephritis. The other causes of death in the prednisolone-only group were esophageal variceal hemorrhage (1 patient), esophageal ulcer hemorrhage (1), hemorrhagic stroke (1), torsade de pointes (1), and terminal liver failure (1); in addition, 1 patient was treated with MARS on day 27. In the prednisolone–N-acetylcysteine group, the other causes of death were esophageal variceal hemorrhage (5 patients) (P=0.11), hemorrhagic stroke (1), and terminal liver failure (1); in addition, 1 patient underwent liver transplantation on day 170.
Adverse Events
At 6 months, the rate of the hepatorenal syndrome was 25% in the prednisolone-only group (22 of 89 patients) and 12% in the prednisolone–N-acetylcysteine group (10 of 85) (odds ratio with combination therapy, 0.41; 95% CI, 0.17 to 0.98; P=0.02) (Table 2). The overall rate of infection was 42% in the prednisolone-only group (37 of 89 patients) and 19% in the prednisolone–N-acetylcysteine group (16 of 85) (odds ratio, 0.33; 95% CI, 0.15 to 0.68; P=0.001). The two groups did not differ significantly with respect to other complications. Among patients with relapse of alcohol use after 1 month, 13% died in the prednisolone-only group (2 of 15) versus 7% in the prednisolone–N-acetylcysteine group (1 of 15) (P=1.00).
Predictive Factors for Death
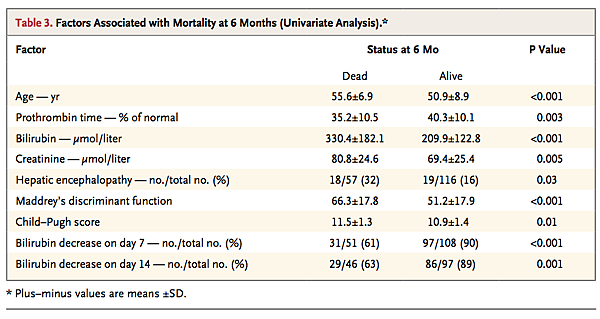
At 6 months, nine factors were significantly associated with mortality in a univariate analysis (Table 3 Factors Associated with Mortality at 6 Months (Univariate Analysis).). Age, hepatic encephalopathy, prothrombin time, baseline bilirubin level, baseline creatinine level, Maddrey's discriminant function, Child–Pugh score, change from baseline in the bilirubin level on day 7, change from baseline in the bilirubin level on day 14, and treatment group were all included in a Cox model for multivariate analysis. Variables independently associated with increased mortality were older age (odds ratio, 1.07; 95% CI, 1.03 to 1.11; P<0.001), prolonged prothrombin time (odds ratio, 0.93; 95% CI, 0.91 to 0.97; P<0.001), higher baseline bilirubin level (odds ratio, 1.007; 95% CI, 1.005 to 1.009; P<0.001), and the absence of a decrease in the bilirubin level on day 14 (odds ratio, 0.23; 95% CI, 0.12 to 0.45; P<0.001).
Change over Time in Bilirubin Level and Effect on Survival
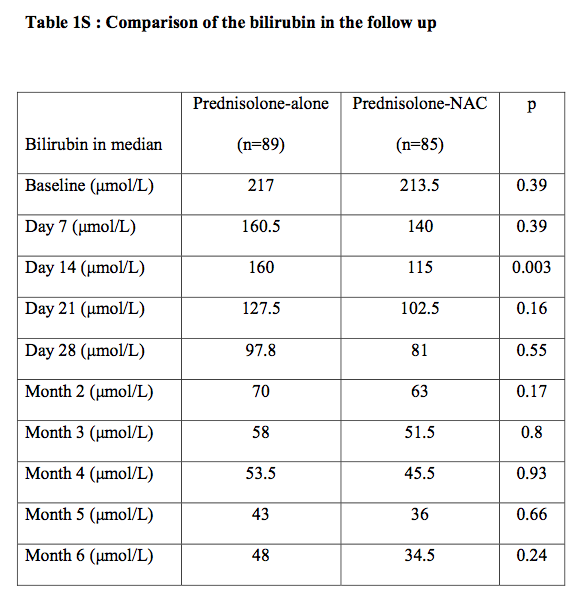
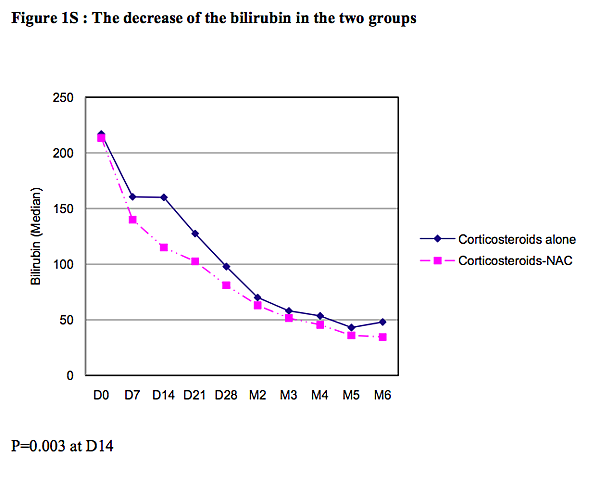
The change in the median bilirubin level in each group over the course of the study is shown in Fig. 1S in the Supplementary Appendix, available at NEJM.org. The difference was significant only on day 14 (160 μmol per liter in the prednisolone-only group vs. 115 μmol per liter in the prednisolone–N-acetylcysteine group, P=0.003) (Table 1S in the Supplementary Appendix).
For the whole study population, data on the bilirubin level on day 7 were available for 159 patients (2 patients had died, and data were missing for 13 other patients). A bilirubin decrease on day 7 was observed in 128 patients (81%). The 6-month survival rate was 90% among patients with a bilirubin decrease on day 7 and 10% among patients without a decrease (odds ratio, 78; 95% CI, 29 to 210; P<0.001). On day 14, data on the bilirubin level were available for 143 patients (7 patients had died, and data were missing for 24 other patients). A bilirubin decrease on day 14 was observed in 80% of the patients. The 6-month survival rate was 89% among patients with a bilirubin decrease on day 14 and 11% among patients without a decrease (odds ratio, 61; 95% CI, 23 to 166; P<0.001). On day 7, 78% of patients in the prednisolone-only group had a bilirubin decrease, versus 81% in the prednisolone–N-acetylcysteine group (odds ratio, 1.38; 95% CI, 0.59 to 3.29; P=0.41). On day 14, 74% of patients in the prednisolone-only group had a bilirubin decrease, versus 87% in the prednisolone–N-acetylcysteine group (odds ratio, 2.47; 95% CI, 0.96 to 6.49; P=0.04).
DISCUSSION
In patients with severe acute alcoholic hepatitis, the combination of N-acetylcysteine and prednisolone did not significantly improve 6-month survival, as compared with prednisolone only. The rationale for the use of antioxidants in the treatment of acute alcoholic hepatitis is based on the pivotal role of oxidative stress in the disorder. Liver protection by N-acetylcysteine has been shown in mouse models of acute and chronic alcoholic hepatitis.20-22 However, in patients with severe acute alcoholic hepatitis, the benefits of antioxidants have not been shown. In a randomized trial, a combination of antioxidants that included N-acetylcysteine was significantly worse than prednisolone with respect to survival.23 Similarly, in a study with a complex design, another N-acetylcysteine-containing antioxidant regimen (with or without glucocorticoids) did not improve 6-month survival.24 Finally, a randomized trial showed that use of N-acetylcysteine for 14 days did not confer any survival benefit, as compared with oral nutritional support.25
Although there was no significant difference in survival at 6 months between our study groups, there was a short-term survival benefit at 1 month with prednisolone–N-acetylcysteine as compared with prednisolone only. We used N-acetylcysteine because it has antioxidant properties,26decreases levels of free radicals, increases glutathione levels,27 and represses the expression of nuclear factor κB and TNF-α.28 The dose, duration, and administration route used were the same as those used for the treatment of drug intoxication29,30 and the hepatorenal syndrome.31 At 3 and 6 months, we observed a lower mortality rate in the prednisolone–N-acetylcysteine group than in the prednisolone-only group, but the differences were not significant. These findings may be related to a lack of power. It is also possible that 5 days of N-acetylcysteine was not enough. A longer period of intravenous N-acetylcysteine combined with prednisolone could perhaps be considered, with subsequent oral administration of N-acetylcysteine until 1 month.
The improvement in short-term survival that we observed in our study could be linked, at least in part, to a reduced risk of the hepatorenal syndrome in the prednisolone–N-acetylcysteine group. In a study involving 12 patients with the hepatorenal syndrome, the survival rate at 1 month after N-acetylcysteine infusion was unexpectedly high (67%).31 In regard to morbidity, the prednisolone–N-acetylcysteine group in our study had significantly fewer infectious complications than the prednisolone-only group. It has been shown that patients with severe alcoholic hepatitis who do not have a response to treatment have significantly more bacterial infections than patients who have a response.32 Alternatively, N-acetylcysteine could have beneficial effects by increasing blood flow to the liver, improving liver function, increasing the cardiac index,33 and decreasing the intrahepatic lactate levels seen in patients with septic shock.34
Our study provides prospective validation of the finding that a decrease in the bilirubin level after 7 days of treatment is associated with a favorable prognosis35 and also shows that a decrease on day 14 is associated with increased survival. However, in a multivariate analysis, only a decrease in the bilirubin level on day 14 remained significant, and the decrease was more frequent in the prednisolone–N-acetylcysteine group than in the prednisolone-only group. Thus, for a 5-day course of prednisolone–N-acetylcysteine therapy, therapeutic efficacy is better evaluated on day 14 than on day 7.
In conclusion, we observed improved survival at 1 month among patients with severe acute alcoholic hepatitis who received combination therapy with prednisolone and N-acetylcysteine, as compared with those who received prednisolone only, but 6-month mortality, our primary outcome, was not improved with combination therapy.





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 