This Journal feature begins with a case vignette that includes a therapeutic recommendation. A discussion of the clinical problem and the mechanism of benefit of this form of therapy follows. Major clinical studies, the clinical use of this therapy, and potential adverse effects are reviewed. Relevant formal guidelines, if they exist, are presented. The article ends with the authors' clinical recommendations.
另外有幾篇也建議妳看看:
Laquinimod (Allegro)– new promising treatment of multiple sclerosis
[Medscape]Fingolimod Receives FDA Approval as First Oral MS Treatment
[Medscape][CME]Emerging Therapies in Multiple Sclerosis
A 37-year-old man with multiple sclerosis was evaluated for consideration of oral immunotherapy. Six years earlier, he had reported acute monocular visual disturbance. The diagnosis of multiple sclerosis was subsequently confirmed by means of examination of cerebrospinal fluid, which revealed an increased IgG index and oligoclonal banding, and by abnormal results on magnetic resonance imaging (MRI). There was no family history of multiple sclerosis. Daily injections of glatiramer acetate were initiated at the time of diagnosis, but two consecutive annual brain MRI scans revealed substantial disease activity. He was then switched to interferon beta therapy, but this treatment was discontinued after the patient had two episodes of acute neurologic worsening. Monthly natalizumab therapy was then considered, but a test for JC virus antibodies was positive. Neurologic examination showed disk pallor and visual acuity of 20/40 in the right eye, partial internuclear ophthalmoplegia, wide-based gait, and posterior column deficits in both legs. The patient was referred to a specialist in multiple sclerosis for consideration of oral fingolimod therapy.
THE CLINICAL PROBLEM
Multiple sclerosis is an inflammatory disease of the central nervous system that shares several genetic variants with other autoimmune diseases. It affects approximately 400,000 people in the United States and 2.5 million people worldwide, with a prevalence of approximately 1 case per 1000 persons in white populations (ratio of females to males, >2:1).1 The vast majority of patients with multiple sclerosis (approximately 85%) have a relapsing form of the disease at onset (relapsing–remitting course). More than half of these patients have increasing and sustained disability between the acute attacks and make a transition to secondary progressive multiple sclerosis within 10 to 20 years after diagnosis.2,3
The life expectancy of patients with multiple sclerosis is reduced; in one study (Canadian registry),4life expectancy was reduced by 4 to 7 years, and in another (Danish registry),5 it was reduced by up to 10 to 12 years. A patient's quality of life is markedly affected by physical symptoms, including fatigue, pain, and difficulty with mobility, and by problems with social functioning and impaired mood and affect. The costs of drugs and other medical care for patients with multiple sclerosis are substantial; in the United States alone, the costs have been estimated at $14 billion per year.6 The total mean annual direct and indirect costs (associated with loss of work and productivity) per patient, in 2004 dollars, are $47,215.
PATHOPHYSIOLOGY AND EFFECT OF THERAPY
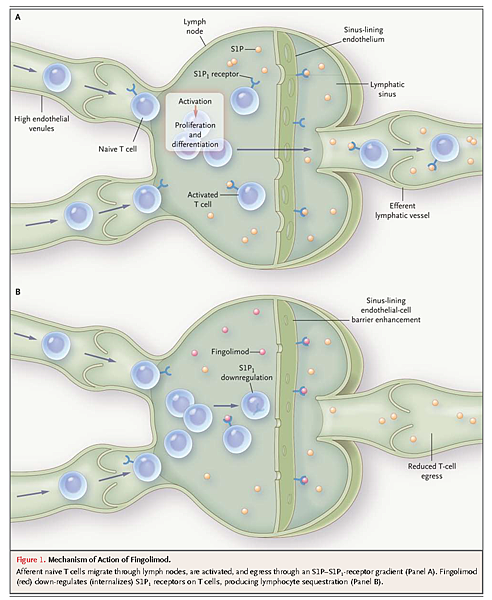
Multiple sclerosis is an autoimmune disease in which lymphocytes migrate out of lymph nodes into the circulation, cross the blood–brain barrier, and aggressively target putative myelin antigens in the central nervous system, causing inflammation, demyelination, neuroaxonal injury, astrogliosis, and ultimately neurodegeneration.7 Lymphocyte migration out of lymph nodes is dependent on engagement of a G protein–coupled receptor, S1P1, which is present on the surface of the lymphocyte.8-10– The ligand for this receptor, the lipid sphingosine S1P, is found predominantly in the serum and lymph. A gradient in the concentration of S1P (low in the lymph node and higher in the circulation) induces the migration of lymphocytes from the lymph node into the circulation (Figure 1A)
Fingolimod, also known as FTY720, is structurally similar to S1P. Fingolimod is phosphorylated in vivo after oral ingestion and can then engage four of the five known S1P receptors (S1P1, S1P3, S1P4, and S1P5). In lymph nodes, fingolimod initially acts as an agonist of the S1P1receptor and then becomes a highly potent functional antagonist, leading to internalization of S1P1 receptors on lymph-node T cells11,12, (Figure 1B). As a result, lymphocytes no longer respond to the gradient of S1P and remain sequestered in the lymph node.
Although patients with multiple sclerosis who are treated with fingolimod have a decrease in the total mean lymphocyte count, the effects on lymphocyte subsets are rather specific: the drug blocks the exit of naive and central memory lymphocytes, but not effector memory T cells, from the lymph node.13,14 This effect appears to be a consequence of the fact that naive T cells and central memory cells, but not effector memory cells, express the chemokine receptor CCR7, which induces homing to the lymph nodes. As a consequence, these cells recirculate to the lymph nodes and are sequestered there, with effector memory cells representing the major T-cell population in the blood of patients being treated with fingolimod. These circulating effector memory cells may have a suppressive function, down-regulating the autoimmune response.15 Thus, fingolimod may work both by sequestering autoreactive T cells in the secondary lymph nodes and by enhancing the functionality and frequency of potent circulating regulatory T cells.
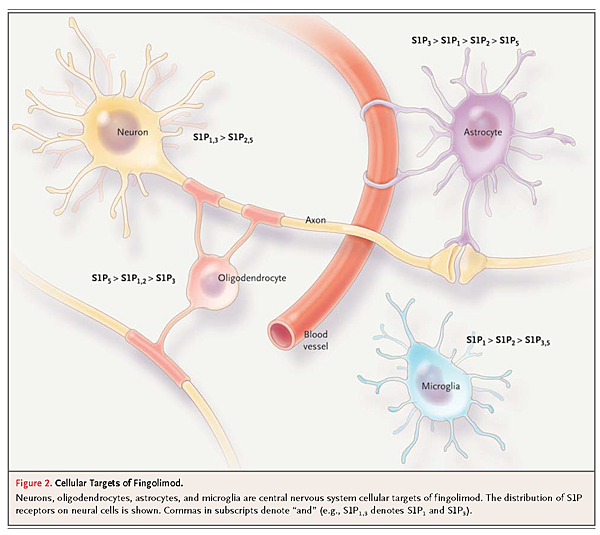
Other studies suggest that fingolimod may also have a direct effect on the central nervous system, since sphingolipids and S1P receptors are expressed on oligodendrocytes, astrocytes, microglial cells, and neurons (Figure 2).
Although this effect is still debated,16 it is possible that fingolimod modulates the survival of human oligodendrocyte progenitor cells, potentially affecting myelin repair17,18 and astrocyte proliferation, migration, and gliosis.19 In addition, the S1P signaling pathway could have neuroprotective effects, as shown in preliminary in vitro studies.20,21
CLINICAL EVIDENCE
The efficacy of oral fingolimod for the treatment of multiple sclerosis was investigated in two large, phase 3, double-blind, randomized trials involving patients with relapsing–remitting disease. Patients with progressive forms of multiple sclerosis were excluded. The FTY720 Research Evaluating Effects of Daily Oral Therapy in Multiple Sclerosis trial (FREEDOMS; ClinicalTrials.gov number, NCT00289978) enrolled 1272 patients who were randomly assigned to receive oral fingolimod at a dose of 0.5 mg daily or 1.25 mg daily or placebo tablets for 2 years.22Neurologic evaluations included measures of acute relapses and disability (as assessed with the use of the Expanded Disability Status Scale [EDSS]). The annualized rate of relapse, which was the primary end point, was 0.18 with the 0.5-mg regimen, 0.16 with the 1.25-mg regimen, and 0.40 with placebo (P<0.001 for the comparison of either regimen with placebo). The cumulative probability of progression of disability (confirmed after 3 months) as assessed on the basis of the EDSS score was 17.7% with 0.5 mg of fingolimod and 16.6% with 1.25 mg of fingolimod, as compared with 24.1% with placebo (P=0.03 and P=0.01 for the comparison of the two fingolimod regimens, respectively, with placebo). Fingolimod was also associated with a reduction in the number of new or enlarging lesions on T2-weighted images and of gadolinium-enhancing lesions on MRI at year 2. Reductions in whole-brain volume were smaller with fingolimod than with placebo at both 12 and 24 months.
The Trial Assessing Injectable Interferon Versus FTY720 Oral in Relapsing–Remitting Multiple Sclerosis (TRANSFORMS, NCT00340834) enrolled 1292 patients who were randomly assigned to receive oral fingolimod at a dose of 0.5 mg daily or 1.25 mg daily or intramuscular interferon beta-1a (30 μg weekly for 1 year).23 Several of the trial participants were already receiving an interferon regimen, which was potentially a confounding factor in this study involving an active comparative intervention, since some patients may have been randomly assigned to a therapy that had already failed to effect a response in them. The annualized relapse rate, the primary end point, was 0.16 in the group that received 0.5 mg of fingolimod, 0.20 in the group that received 1.25 mg of fingolimod, and 0.33 in the interferon group (P<0.001 for the comparison of either fingolimod regimen with interferon). No differences were found with respect to confirmed progression of disability, as assessed with the use of the EDSS. MRI results supported the primary findings.
CLINICAL USE
In September 2010, the Food and Drug Administration (FDA) approved fingolimod (0.5 mg given orally once a day) as a treatment aimed at reducing the frequency of clinical exacerbations and delaying the progression of physical disability in patients with relapsing forms of multiple sclerosis. No formal contraindications are described. Fingolimod was the first oral therapy approved by the FDA for the treatment of multiple sclerosis.
Although fingolimod has been approved as a first-line drug for the treatment of multiple sclerosis, we strongly believe that this agent should be reserved as a second-line drug until further long-term safety investigations have allowed for the evaluation of the risks of infectious complications and secondary cancers (see the section below on adverse effects). A substantial number of patients with multiple sclerosis have a good response to first-line drugs that have minimal side effects; therefore, it seems prudent to initiate treatment with these drugs at the first evidence of a clinically isolated syndrome in a patient with abnormal MRI findings that are consistent with a demyelinating process (Figure 3).25-28–
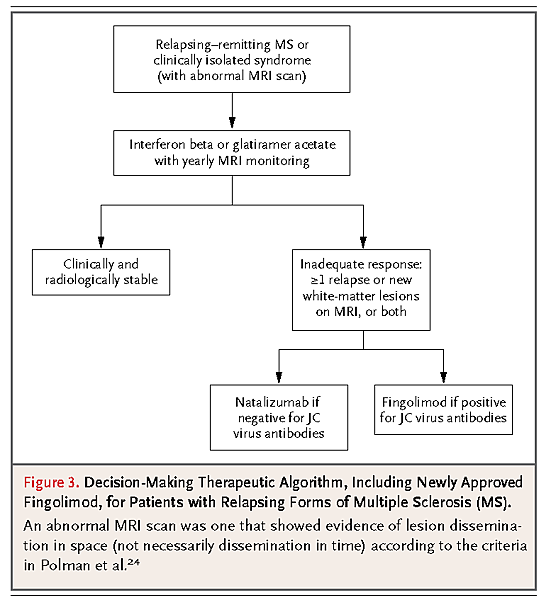
However, considering the apparent benefits from the greater clinical efficacy of the agents affecting T-cell migration (natalizumab and fingolimod) and the consequences of chronic inflammation of the central nervous system associated with multiple sclerosis, we recommend a relatively low threshold for initiating second-line therapies when permanent clinical signs of central nervous system injury are present.Natalizumab therapy has been shown to be associated with an increased risk of progressive multifocal leukoencephalopathy, and this risk has been shown to correlate with the presence of anti–JC virus antibodies.29-31Therefore, we recommend that the choice between natalizumab and fingolimod be based principally on the results of tests for these antibodies.
We further recommend that intravenous immunosuppressive agents (e.g., cyclophosphamide), monoclonal-antibody therapies (e.g., rituximab or alemtuzumab), or other maintenance oral immunosuppressants (e.g., methotrexate, mycophenolate, or azathioprine), which are not FDA-approved for the treatment of multiple sclerosis, should now be considered as third-line options that would be appropriate for consideration only after the patient has had an inadequate response to or side effects from fingolimod and natalizumab. Mitoxantrone, an approved therapy that is still used by some clinicians specializing in the care of patients with multiple sclerosis, is no longer a treatment option in our clinical practice, mainly because of the long-term risk of leukemia associated with this agent.32
Before the initiation of fingolimod therapy, several specific laboratory tests and other assessments should be performed. These include measurement of total and differential leukocyte counts, aminotransferase levels, bilirubin levels, and varicella–zoster antibody titers (if negative, vaccination 1 month before initiation of fingolimod therapy should be considered); electrocardiography (especially if any personal or familial cardiovascular risk factors are present); and spirometry (if pulmonary risk factors are present). For patients who present with electrocardiographic abnormalities, referral to a cardiologist is recommended before the initiation of fingolimod therapy. Special attention should be given to patients who have evidence of atrioventricular conduction block and to patients who are taking drugs known to alter conduction (e.g., psychotropic agents, beta-blocker drugs, and calcium-channel blockers).33 Referrals to ophthalmologists and dermatologists are recommended, at least until the actual risk of macular edema and skin cancer is known (see the section on adverse effects).
No adjustments of the dose of fingolimod are necessary in patients who have mild or moderate hepatic or renal impairment or in those who are elderly. However, caution is warranted in these populations. Patients undergoing dialysis or plasmapheresis do not have enhanced removal of fingolimod from the blood, and therefore do not require dose adjustment.
The patient's vital signs must be monitored for 6 hours after the first oral dose is administered, to detect any decrease in the heart rate. The drug should be discontinued if bradycardia is documented. The observation of vital signs does not require hospitalization and can be performed in an outpatient clinical office. No other immediate reactions have been reported. The patient should then be reevaluated clinically every month to assess the response to treatment, as well as to observe any side effects. Measurement of leukocyte counts and aminotransferase and bilirubin levels should be repeated every 3 months. A repeat ophthalmologic evaluation should be performed 3 months after the initiation of fingolimod therapy and every 6 months thereafter; annual dermatologic evaluations are also recommended.
As with several other FDA-approved therapies for multiple sclerosis, the safety and efficacy of fingolimod have not been evaluated formally in children. In addition, there are no data from adequately controlled studies of fingolimod therapy in pregnant women; therefore, it is a pregnancy category C drug, and we do not recommend its use during pregnancy. Women with multiple sclerosis who could become pregnant should use effective contraception during treatment and for 2 months after discontinuation of treatment. It has been shown that fingolimod is excreted in the milk of rodents; therefore, the drug should be discontinued for at least 2 months, or not initiated at all, in women who wish to breast-feed.
Discontinuation of fingolimod, for reasons such as pregnancy, lack of therapeutic response, or undesirable side effects, does not require tapering of the dose. If a patient interrupts fingolimod therapy for more than 2 weeks and then resumes treatment, monitoring of vital signs for 6 hours is again necessary.
The yearly wholesale price of fingolimod, as currently listed, is approximately $52,200.34 Additional costs for services related to administration of the first dose (e.g., 6-hour monitoring of vital signs), referral visits, and tests associated with monitoring vary considerably according to the local medical care environment and geographic area.
ADVERSE EFFECTS
The safety profile of oral fingolimod therapy in patients with multiple sclerosis is derived from large pharmacologic databases and from phase 2, phase 3, and extension studies.22,23,35,36Furthermore, a risk-evaluation and mitigation strategy (REMS) has been developed to educate patients and providers about the long-term risks. However, unlike the case with natalizumab (and perhaps unfortunately), there is no mandatory monitoring program for fingolimod. It is important that clinicians familiar with fingolimod and the potential complications associated with it monitor patients and actively report cases of unexpected adverse events.
One death has been reported in the post-marketing period after the administration of a single dose of fingolimod.37 As of this writing, the exact cause of death has not been determined, and the role of fingolimod can neither be confirmed nor ruled out.
There were two deaths reported during the TRANSFORMS trial, both in the group assigned to the higher-dose fingolimod regimen. In one of these patients, a primary disseminated herpes zoster infection developed after the patient was exposed to chicken pox during the course of intravenous methylprednisolone treatment for a relapse of multiple sclerosis; the second patient had herpes simplex encephalitis. It is not clear whether fingolimod contributed to the deaths, since both cases involved the use of glucocorticoids in the context of a clinical relapse; however, given the mechanism of action of fingolimod, it is highly plausible that these deaths were attributable to fingolimod therapy. There was also a slight increase in the incidence of mild infections, mainly infections of the lower respiratory tract (bronchitis and pneumonia) (FREEDOMS study) and herpesvirus infections (primarily among patients receiving 1.25 mg of fingolimod).
The most common serious adverse effects reported in previous studies were cardiovascular events, including bradycardia (in 1 to 3% of patients) and first-degree or second-degree atrioventricular block (in <1%). Most of these events were asymptomatic, were observed during the administration of the first oral dose, resolved within 24 hours, and did not recur despite the continuation of therapy. These changes probably reflect the effects of fingolimod on the S1P receptors on atrial myocytes.38 In addition, a mild dose-dependent increase in systolic and diastolic blood pressure was observed over the course of 2 years.
Macular edema was confirmed by ophthalmologic evaluation in less than 1% of the patients. Most of the cases of macular edema were asymptomatic and resolved within months after discontinuation of fingolimod therapy.
Localized skin cancers (basal-cell carcinoma and melanoma)39 and breast cancers were reported more frequently in the groups receiving either high-dose or low-dose fingolimod than in the group receiving interferon beta-1a in the TRANSFORMS trial. Long-term follow-up studies are needed to clarify the actual risk of cancer.
There were mild dose-dependent decreases in lung function, as assessed by means of forced expiratory maneuvers; decreases in exhaled volumes were observed within the first month after the initiation of therapy, and there were no subsequent reductions. These effects on pulmonary function could reflect the mechanism of action of fingolimod on the S1P receptors on smooth-muscle cells and endothelial barrier cells.40
As expected, peripheral-blood lymphocyte counts were reduced by approximately 75% from baseline after the first month. Asymptomatic elevations in liver enzyme levels (e.g., alanine aminotransferase) were seen more frequently in patients receiving fingolimod than in those receiving either interferon beta-1a or placebo. Enzyme levels returned to the normal range in all patients, even in those who continued to take fingolimod.
Fingolimod has been shown to be teratogenic in rodents.41 In humans, several pregnancies have been reported among women receiving fingolimod, but because the number of pregnancies is low, no firm conclusions can be drawn. The establishment of a pregnancy registry should help define the risk of fingolimod to fetuses.
AREAS OF UNCERTAINTY
The risks of adverse effects with fingolimod, especially in the long term, remain uncertain. The risks include severe infections, such as disseminated and central nervous system herpetic infection; effects on immune surveillance (a possible increase in the risk of progressive multifocal leukoencephalopathy, toxoplasmosis, or neoplasms); and effects on the reproductive system after long-term use. Long-term (5-to-10-year) controlled extension studies are necessary, since such risks cannot be addressed adequately in short-term studies.
It remains unclear whether fingolimod has a direct effect on oligodendrocytes, astrocytes, and neurons in vivo (as suggested in Figure 2). If so, fingolimod could have the potential to offer primary neuroprotection and help prevent further disability. To address this issue, fingolimod is currently being evaluated in a 3-year, multicenter, phase 3 study involving patients with primary progressive multiple sclerosis.42,43
Additional direct comparisons of fingolimod with other available therapies for multiple sclerosis are crucial. For example, clinicians must often decide between natalizumab and fingolimod for patients who are having suboptimal responses to current injectable therapies. Establishing the minimal effective dose of fingolimod in clinical trials could also minimize the risk of adverse events and improve the risk-to-benefit ratio.
GUIDELINES
Currently, there are no established clinical guidelines, published by national or international neurologic societies, for the use of fingolimod in patients with multiple sclerosis. We believe that until additional long-term safety data are available, fingolimod therapy should be considered only for patients who have had recent inflammatory disease activity (one or more relapses or new white-matter lesions on MRI within the past year) and those who do not benefit from or cannot tolerate alternative disease-modifying therapies. We also recommend that patients not have taken immunosuppressive medications or received a natalizumab infusion within 3 months before the initiation of fingolimod therapy (6 months if the patient has received long-term intravenous immunosuppression with agents such as mitoxantrone or cyclophosphamide). No specific washout period is needed after cessation of glatiramer acetate or beta-interferon therapy. Other FDA-approved medications for the treatment of multiple sclerosis should not be initiated until 2 months after discontinuation of fingolimod therapy, to allow for elimination of fingolimod and normalization of lymphocyte counts and to minimize the risk of additive immunosuppressive effects. Until additional safety data are available, patients receiving fingolimod therapy who have lymphocyte counts of less than 200 cells per cubic millimeter should discontinue therapy; resumption of therapy may be considered after normalization of the lymphocyte count.





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 