Abstract
Over the last decade, advances in diagnostic systems and the introduction of new antifungal agents have significantly improved outcomes in immunocompromised patients who develop invasive aspergillosis. However, mortality rates remain relatively unchanged for less common, but highly aggressive, mold infections such as mucormycosis. Recent genome sequencing of Rhizopus oryzae revealed evidence of a whole-genome duplication event during the evolution of this pathogen. Consequently, R. oryzae has a 2- to 10-fold enrichment in gene families associated with ergosterol and cell wall biosynthesis, cell growth, iron uptake, and known fungal virulence factors compared with sequenced Aspergillus fumigatus strains. This genetic plasticity may explain the remarkable capability of this pathogen for rapid growth in hostile environments, such as the inflammatory milieu, as well as its relative resistance to multiple antifungal classes. Herein, we examine how pharmacological aspects of treating mucormycosis may differ from those of the more commonly encountered invasive aspergillosis.
The prognosis of opportunistic mold infections in severely immunocompromised patients is significantly better than a decade ago [1]. Advances in diagnostic imaging, serum-based detection of fungal pathogens, and the introduction of safer, broad-spectrum antifungal agents have coincided with declining mortality rates of invasive aspergillosis in the hematologic-malignancy population [2, 3]. Yet in a subset of patients with profoundly weakened immune function, a second wave of insidious but often aggressive fungal pathogens (Mucorales, Fusarium species, and Scedosporium species) may supplant Aspergillus species, breaking through antifungal therapy and spreading to vital organs before the infection is suspected. The incidence of mucormyosis has increased nationwide between 1997 and 2006, especially in the hematology patient population and is now the second most common invasive mold infection (after invasive aspergillosis) diagnosed among patients who undergo hematopoetic stem cell transplantation in the United States [4, 5]. Unlike aspergillosis, the majority of hematology patients who develop mucormycosis still die from their infection despite the administration of systemic antifungal therapy, emphasizing the critical need for new treatment approaches. In this article, we will discuss recent data concerning antifungal pharmacology for mucormycosis that have emerged from genome sequencing efforts, as well as in vitro and in vivo studies of antifungal therapy. Specifically, we will explore the reasons for which many antifungal agents are less effective for the treatment of mucormycosis compared with aspergillosis.
INSIGHTS INTO ANTIFUNGAL TARGETS FROM GENOME SEQUENCING OF RHIZOPUS ORYZAE
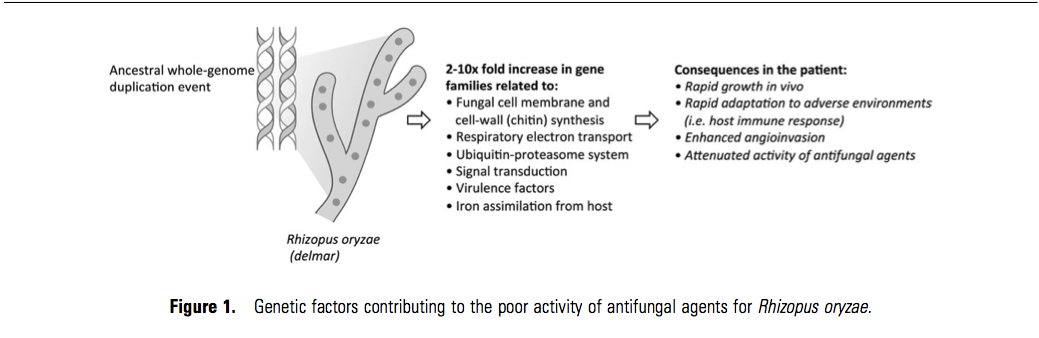
Rhizopus oryzae is a rapidly growing, filamentous fungus that ranks among the most common causes of invasive mucormycosis [6]. The recent sequencing of aR. oryzae strain isolated from a patient with a fatal infection revealed a surprising number of repetitive gene families relative to other sequenced pathogenic fungi that may have resulted from an ancestral whole-genome duplication event followed by a massive gene loss [7]. This evolutionary path appears to have dramatically enriched the R. oryzae genome with the capabilities for maintaining growth and metabolism under highly varied environmental conditions, production of fungal virulence factors (eg, secreted aspartic proteases and subtilases [8, 9]), capabilities for accelerated fungal cell wall/membrane synthesis and remodeling, and iron assimilation from host hemoglobin [10, 11] (Figure 1). Consequently, R. oryzae is genetically equipped for rapid angioinvasive growth in humans, adaptation to hostile environments, such as the host immune response, and overcoming the effects of systemically administered antifungal agents.
Ergosterol Biosynthesis and the Cell Membrane
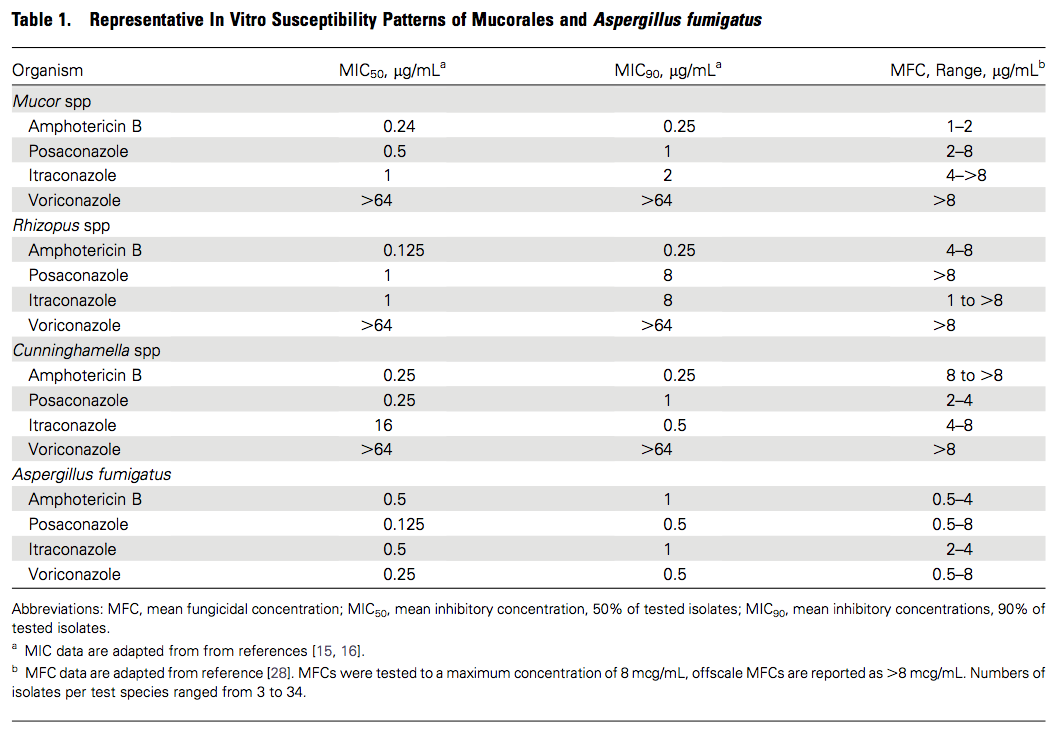
In terms of the classic antifungal targets, the ergosterol biosynthesis pathway is conserved in the R. oryzae genome, supporting the concept that polyene and triazole antifungal agents that target the enzymes or products of this pathway may be therapeutically useful for mucormycosis [12]. However, approximately one-half of the genes involved in ergosterol biosynthesis, including the principal fungal target of the triazoles, 14α-demethylase (ERG11), are present in multiple copies [7]. Because acquisition of azole resistance in other fungal species has been associated with amplification of ERG11 in a gene-copy-dependent manner [13, 14], it is possible that the increased copy number and divergence of duplicated ERG11 protein sequences in R. oryzae may contribute to the variable responses of this fungus to voriconazole, itraconazole, and posaconazole [7]. Minimum inhibitory concentration values for itraconazole and posaconazole are often 4–8 dilutions higher for R. oryzae compared withAspergillus fumigatus, and fungicidal activity is not observed for many isolates over a range of clinically achievable serum drug concentrations [15–17] (Table 1). Voriconazole lacks clinically useful activity against R. oryzae, as evidenced by the frequent breakthrough infections in patients receiving voriconazole therapy [19, 20]. In comparison to aspergillosis, relatively little is known about the pharmacodynamic dosing targets required with triazole or polyene antifungal agents for effective treatment of mucormycosis [21]. Furthermore, mechanisms of adaptive resistance or cross-resistance to triazole and polyene antifungal agents have not been systematically studied [22]. Given the greater genomic plasticity of R. oryzae relative to A. fumigatus, the potential for development of resistance during long-term triazole therapy may be higher in patients with mucormycosis.
Amphotericin B has long been considered the mainstay therapy for mucormycosis, and, if administered early, can significantly decrease the risk of fungal dissemination and patient death [6, 19, 23, 24]. Most clinicians use a lipid formulation of amphotericin B with a usual starting dose of 5 mg/kg/day that is sometimes escalated to 10 mg/kg/day (liposomal amphotericin B) in an effort to control the infection. Recently, the use of lipid formulations of amphotericin B has been associated with a better outcome of mucormycosis occurring in solid organ transplant patients [20]. High-dose liposomal amphotericin B carries a greater risk for nephrotoxicity and was not shown to be more effective than a 3 mg/kg daily dose in leukemic patients with predominantly invasive pulmonary aspergillosis [25]. Nevertheless, animal models have suggested that higher amphotericin B tissue concentrations may be required early during the infection for effective treatment of mucormycosis versus aspergillosis [18, 26, 27], which would be consistent with the relative tolerance in vitro of R. oryzae and Cunninghamella species to the fungicidal effects of amphotericin B [28] (Table 1). Currently, a phase 2 clinical study (AmbiZygo) of initial 10 mg/kg/day liposomal amphotericin B is under way for mucormycosis regardless of the underlying disease and age (http://clinicaltrials.gov/ct2/show/NCT00467883).
The optimal dosage for treatment of invasive pulmonary, sinus, and rhinocerebral mucormycosis is not known. Each site of infection presents different pharmacodyamic challenges. It is important for lipid formulations of amphotericin B to be able to distribute into pulmonary alveolar macrophages and epithelial lining fluid in lung tissue [29]. By comparison, paranasal sinuses have respiratory epithelium but no residual macrophage population. Rhinocerebral infections entail a need to penetrate into central nervous system tissue [30]. Development of plasma pharmacokinetic models for amphotericin B lipid formulations are also complicated by multicompartmental disposition profiles [31], nonlinearity [32], and dose-dependent clearances [33]. For example, the area under the concentration–time curve at 24 hours of liposomal amphotericin B increases in a nonlinear pattern from 1.0 mg/kg to 10.0 mg/kg in patients and then declines at dosages of 12.5 mg/kg and 15.0 mg/kg. Considerably less is known about the human pharmacology of amphotericin B lipid complex at 5 mg/kg, which is the most widely reported dosage. While the dosages between 5.0 mg/kg and 10 mg/kg of amphotericin B lipid formulations are used in the treatment of mucormycosis, the optimal dosage for exposure and safety for these conditions is not known.
Ultimately, combination therapy may be a safer strategy than dosage escalation for improving the activity of amphotericin B–based therapy for mucormycosis. Combinations of a standard-dose lipid formulation of amphotericin B plus either an echinocandin [34, 35], a newer-generation iron chelation agent (deferasirox) [36, 37], or other adjunctive therapies may lower the amphotericin B concentrations required for fungicidal effects in vivo. The role of combination therapy for mucormycosis is discussed elsewhere in the supplement.
Fungal Cell Wall
Compared to Aspergillus species, the cell wall of R. oryzae and other Mucorales members contains a high percentage of chitin and chitosan, which are synthesized by chitin synthases (CHS) and chitin deacetylase (CDA) [38]. The R. oryzae genome contains more than double the number of CHS and CDA gene families compared with that of A. fumigatus, with the majority of chitin synthases transcriptionally active during growth [7]. However, genes encoding glucan synthases are relatively underrepresented relative to A. fumigatus, as R. oryzae contains only 2 copies of (1→3)-β-D-glucan synthase, and no gene encoding (1→3)-α-D-glucan synthase [7].
Although echinocandins display virtually no activity in vitro against Mucorales, they are modestly effective in vivo in the treatment of experimental disseminated mucormycosis. Ibrahim and colleagues [39] reported that the FKS1(1→3)-β-D-glucan synthase in R. orzyae displayed >70% sequence homology toFKS1 in A. fumigatus, and crude membrane preparations of glucan synthase from R. oryzae were inhibited by caspofungin, albeit at higher concentrations than for A. fumigatus (Inhibitory concentration 50% 11 900 ng/mL vs 0.24 ng/mL). Interestingly, the investigators found that lower-dose caspofungin monotherapy (0.5 mg/kg twice daily) significantly prolonged the survival of diabetic ketoacidotic mice intravenously infected with low inoculums of R. oryzae spores (80% survival), but higher-dose caspofungin regimens were not statistically better than the control (40% survival) [39]. Similar patterns were observed with other echinocandins used alone and in combination with lipid amphotericin B and in a neutropenic pulmonary model of mucormycosis [40,41]. The attenuated activity of echinocandins at higher daily doses may reflect upregulation of homeostatic cell-wall responses in the fungi that “rescue” the fungus from the effects of echinocandins through compensatory increases in chitin synthesis [42]. This response may be more robust in R. oryzae versus A. fumigatus considering the expanded number of Rhizopus gene families associated with signal transduction, cell wall biogenesis, and chitin synthesis [7,43]. It is also possible that some of the activity observed with echinocandins in animals models stems from immunopharmacological mechanisms of drug activity in vivo [44]. Exposure of R. oryzae germlings to echinocandins has been shown to increase the surface exposure of immunogenic β-glucans in hyphae, resulting in enhanced human polymorphonuclear cell damage ex vivo [45]. However, increased β-glucan release could also theoretically enhance immunopathology in the lung through TH-17-dependent mechanisms [46]. Therefore, it is unclear if echinocandins would be equally effective in all types of immunosuppression backgrounds encountered in patients with (pulmonary) mucormycosis and which dosage should be used in a future clinical trial.
PHARMACOKINETIC CHALLENGES OF TREATMENT FOR MUCORMYCOSIS
Clinical trials of aspergillosis treatment performed in the last decade clearly demonstrated the importance of timing in the ability of antifungal therapy to improve survival. Caillot and colleagues [47] and Greene et al [48] found that treatment initiated at the time of the earliest suggestive radiographic manifestations of invasive pulmonary aspergillosis, such as the halo sign, was associated with significantly better treatment response and survival than in patients who had antifungal therapy initiated with computed tomographic imaging findings that predominated 7–10 days later. Treatment delays allow unimpeded angioinvasive fungal proliferation with thrombotic necrosis of surrounding tissue that limits delivery of life-saving drugs to the sites of infection [49–51]. Due to the rapid growth rate of most Mucorales in vivo, the “window of opportunity” for effective treatment of mucormycosis (ie, before angioinvasion and dissemination becomes too extensive) may be much shorter than that for patients with aspergillosis. The importance of timely diagnosis and early treatment was illustrated in a retrospective study of 70 leukemia patients with documented mucormycosis. Delays in the administration of amphotericin B–based regimens by >5 days was associated with nearly a 2-fold increase in mortality at 12 weeks after diagnosis (83% vs 49%; P = .03) [24]. Similarly, in a recent retrospective study, Lass-Florl and colleagues reported that 84% of patients with pulmonary zygomycosis were receiving ineffective therapy at the time diagnosis was established by histopathology [52]. Therefore, treatment strategies during the initial phases of the infection should emphasize the use of pharmacokinetically predictable drugs (ie, lipid amphotericin B formulations with or without echinocandins) to quickly load infected tissue with drug so that fungal proliferation is slowed and the risk of further angioinvasion is reduced. Once the infection has stabilized, greater emphasis can be placed on the convenience of oral therapy using drugs with improved safety profiles such as posaconazole, whose pharmacokinetic variability can be optimized through careful clinical assessment of gastrointestinal disturbances, diet optimization, and therapeutic drug monitoring. However, the use of posaconazole for primary treatment of mucormycosis remains investigational and should be done with an abundance of caution, owing to the inconsistent activity reported in several studies of experimental disseminated mucormycosis [53].
NEW DRUG TARGETS FOR MUCORMYCOSIS?
The sequencing of the R. oryzae genome has provided not only a clearer picture of why antifungals may be less lethal for this fungus, but also insight into potential strategies for improving antifungal activity. Chitin synthesis would seem to be an obvious target for a drug that can be used against Rhizopus.Although a number of chitin-synthesis inhibitors have been identified and well characterized since the 1970s (ie, polyoxins and nikkomycins), their development as effective therapeutic agents has been limited by their poor uptake in intact fungal cells and the differential susceptibilities of CHS gene products to the inhibitory effects of these antifungals [55]. These challenges could be especially problematic for Mucorales, given the duplicative and highly redundant nature of chitin synthesis in these fungi [7]. This was confirmed, in part, by in vitro studies that examined combinations of micafungin and the chitin synthesis inhibitor nikkomycin Z, and reported synergistic interactions forA. fumigatus isolates but not for R. oryzae [56, 57]. However, synergistic interactions have been observed in vitro when amphotericin B or triazoles (posaconazole, itraconazole, or ravuconazole) are combined with a calcineurin inhibitor such as cyclosporine against several Mucorales members [57]. Simultaneous targeting of the cell membrane, cell wall, and signal transduction pathways involved in fungal homeostatic responses (eg, protein kinase C or the calcineurin pathway) may prove to be an important strategy for improving drug activity in this remarkably adaptive pathogen.
Genomic analysis of R. oryzae further confirmed the unique importance of free iron in the pathogenesis and growth of this aggressive pathogen [7]. Unlike other microbes, R. orzyae lacks genes for nonribosomal peptide synthases, which are the enzymes that produce the most common siderophores (hydroxamate siderophores). Instead, R. oryzae relies solely on rhizoferrin, which is ineffective at acquiring serum-bound iron [58] and is therefore dependent on free iron or the actions of iron reductases (eg, rFTR1) and hemeoxygenases to increase iron uptake from host hemoglobin. Novel inhibitors of iron uptake in R. oryzae could impair the capacity of this fungus for angioinvasive growth. This critical role of iron uptake during R. oryzae infection also reinforces the promise of using novel iron chelators such as deferasirox and deferiprone to improve outcomes in patients with mucormycosis, although many questions remain regarding with which companion drug it should be administered, as well as the optimal timing, dosing, and duration of these novel therapies for mucormycosis.
Notes
Supplement sponsorship.
This article was published as part of a supplement entitled “Advances Against Mucormycosis: A Tribute to the Memory and Courage of Hank Schueler,” sponsored by the Henry Schueler 41&9 Foundation.
Potential conflicts of interest.
R. L. has received grant support from Merck & Co, Gilead, Astellas, and Enzon. O. L. has received grant support from Gilead and Astellas; has been a consultant for Astellas; and has served on the speakers’ bureaus of Astellas, Gilead, Pfizer, Schering-Plough, and Merck. B. S. has received grant support from Gilead, Astellas, and Novartis; has been a consultant for Merck, Pfizer, Arpida, Theravance, Advanced Life Sciences, Basilea, The Medicines Company, Novo Nordisk, Novartis, and Cerexa; and is a shareholder of NovaDigm Therapeutics and Neutropenia Immunotherapy Solutions. T. J .W. has received grant support from Novartis and Astellas and has been a consultant for Trius, iCo, Sigma Tau, Draius, and Novartis. D. K. has served on the boards of and received consultancy support from Schering-Plough and Merck. E. R. has been a consultant for Schering, Gilead, Astellas, Cephalon, Pfizer, Wyeth, Merck, and Aventis and has served on the lecture/speakers’ bureaus of Pfizer, Gilead, Enzon, Schering, and Wyeth.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.






 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 