如果怕文章太長,可以直接到這邊查資料庫:Drugs that Prolong the QT Interval and/or Induce Torsades de Pointes
Mechanisms and Clinical Management
Abstract and Introduction
Abstract
The prolonged QT interval is both widely seen and associated with the potentially deadly rhythm, Torsades de Pointes (TdP). While it can occur spontaneously in the congenital form, there is a wide array of drugs that have been implicated in the prolongation of the QT interval. Some of these drugs have either been restricted or withdrawn from the market due to the increased incidence of fatal polymorphic ventricular tachycardia. The list of drugs that cause QT prolongation continues to grow, and an updated list of specific drugs that prolong the QT interval can be found at www.qtdrugs.org. This review focuses on the mechanism of druginduced QT prolongation, risk factors for TdP, culprit drugs, prevention and monitoring of prolonged drug-induced QT prolongation and treatment strategies.
QT Interval Physiology and Mechanism of QT Drug-induced Prolongation
The QT interval on the surface EKG represents the summation of action potential (AP) of ventricular myocytes. The action potential reflects the flow of ion currents across a cell membrane through specialized channels made of protein complexes (Figure 1, Titier et al. 2005). Malfunction of these protein channels can lead to either increased inward current or reduced outward current. This subsequently increases the action potential duration and hence QT interval prolongation.
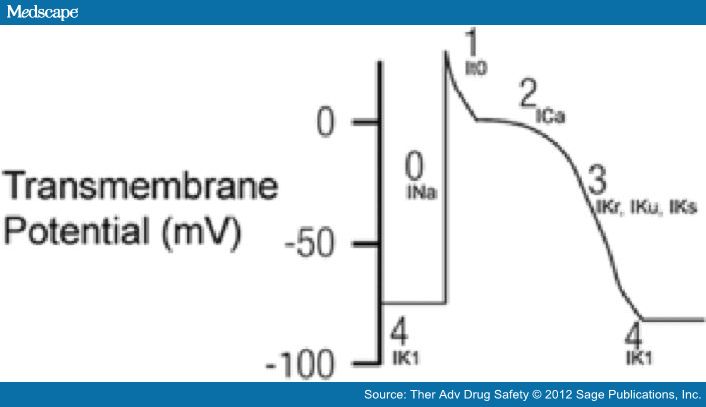
Figure 1.
Five phases of cardiac depolarization and repolarization. Phase 0: Large inward current of sodium ions (INa). Phase 1: Inactivation of INa and the transient efflux of potassium ions (It0). Phase 2: Plateau phase with influx of calcium ions through L-type calcium channels (ICa) and outward repolarizing potassium currents (IK). Phase 3: Efflux of potassium (IKr, IKu, IKs). Phase 4: Inward rectifier potassium current (IK1) maintaining resting potential. ICa, calcium current; IK, potassium current; IK1, inwardly rectifying potassium current; INa, depolarizing sodium current; It0, transient outward potassium current; IKr, rapidly activating delayed rectifier potassium current; IKs, slowly activating delayed rectifier potassium current. (Reproduced with permission from Titier et al. [2005].)
Mutations of the genes that encode the protein channels (IKr, IKs and Na) result in congenital long QT syndrome (LQTS) [Ching and Tan, 2006]. In acquired LQTS, the mechanism is almost always due to blockage of the inward potassium rectifier (IKr) channel, also known as the hERG (ether a go go) channel. It conducts a rapid delayed rectifier potassium current (Ikr), a critical current in the phase 3 repolarization of the cardiac action potential [Roden and Viswanathan, 2005]. Inherited mutations (loss of function) of the hERG gene lead to type 2 LQTS. Medications that prolong QT interval act on the same hERG channel. The distinct molecular structure of the hERG channel makes it more susceptible to medications.
The Structure of the Herg Channel
The structure of the hERG channel is well understood from the structure of bacterial and mammalian K channels [Swartz, 2004]. The hERG channel is essentially formed by the co-assembly of four alpha subunits, each of which has six transmembrane spanning alpha-helical segments (S1–S6) [Sanguinetti and Tristani-Firouzi, 2006; Swartz, 2004] (Figure 2). The first four helices (S1–S4) in each segment form a voltage sensor domain (VSD) that senses the transmembrane potential. The next two helices (S5 and S6) form the pore domain that contains a short alpha helix (pore helix) and selectivity filter. Four of these (one from each subunit) come together to form a central pore that is responsible for the movement of potassium current. Below the selectivity filter, the pore widens to form a central cavity. It is lined by many unique aromatic residues that are absent in most other K channels. These optimally positioned aromatic residues and the polar residues are an integral part of the unique binding sites for diverse pharmacologic agents [Perry et al. 2010; Perrin et al.2008; Kannankeril, 2008].
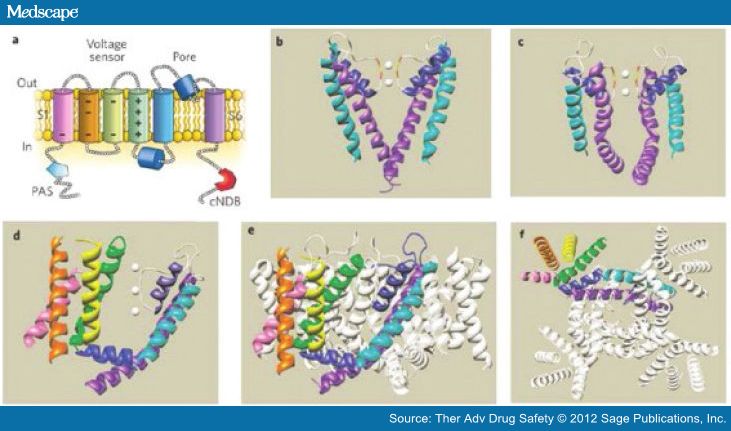
Figure 2.
(a) A single hERG subunit containing six α-helical transmembrane domains, S1–S6. (b) Structure of a KcsA K+ channel crystallized in the closed state. Only two of the four subunits are shown. White spheres are K+ ions located within the selectivity filter. The Gly (red) and Tyr (yellow) residues of the selectivity filter are also indicated. (c) Structure of the pore domain of a Kv1.2 K+ channel crystallized in the open state. Only two of the four subunits are shown. (d) Crystal structure of a single Kv1.2 α-subunit7 viewed from the side. Color coding of the helical domains is the same as in panel (a). Grey spheres represent K+ ions. (e) Side view and (f) view from the cytoplasmic side of the membrane of the crystal structure of the complete, tetrameric Kv1.2 channel. (Reproduced with permission from Sanguinetti and Tristani-Firouzi [2006].)
The other mechanisms by which drugs induce prolonged QT interval are: disruption of KCNH2 protein trafficking leading to loss of K channels by drugs such as arsenic oxide, pentamidine and fluoxetine [Ficker et al. 2004; Kuryshev et al. 2005; Rajamani et al. 2006], rescue of SCN5A channel causing increased inward sodium current by cisapride [Kannankeril, 2008] and an increase inward calcium current by antimony [Kuryshev et al. 2006].
The Congenital Long QT Syndrome
More than 10 different types of congenital LQTS have been recognized [Hedley et al. 2009; Modell and Lehmann, 2006; Roden, 2008]. LQT1, LQT2, and LQT3 account for the majority of the cases of congenital LQTS. LQT1 accounts for 40–55% of cases of the LQTS [Schwartz et al. 2001; Splawski et al.2000]. It is caused by mutations in the KVLQT1 (also called KCNQ1). LQT1 is characterized by events that are induced by exercise.
LQT2 accounts for 35–45% of cases of congenital LQTS [Schwartz et al. 2001; Splawski et al. 2000]. It is caused by a variety of mutations in the hERG (also known as KCNH2) potassium channel gene, located on chromosome 7. The mutations may involve the pore or the nonpore region of the hERG channel. Pore mutations carry high risk for cardiac events and may affect young patients [Moss et al. 2002] whereas nonpore mutations often lead to Torsades de Pointes(TdP) in the presence of hypokalemia [Berthet et al. 1999]
LQT3 accounts for 8–10% of cases [Schwartz et al. 2001; Splawski et al. 2000]. It is caused by mutations in the sodium channel gene (SCN5A) located on chromosome 3 at location 21–24. It is characterized by events occurring at rest or during sleep.
Measurement of the QT Interval
On a 12-lead ECG, the QT interval is measured from the beginning of the QRS complex to the end of T wave as it returns to baseline. Manual measurements of the QT interval should be taken from leads II and V5 or V6 with the longest value being used. Measurements taken from these leads have the greatest positive and negative predictive value in detecting abnormal QT intervals [Monnig et al. 2006]. A mean value should be derived from at least 3–4 cardiac cycles. The end of T wave can be determined reliably by the slope method where it is defined by the intersection point between the tangent drawn at the maximum downslope of the T wave and the iso-electric line. If the T wave is notched, the tangent should be applied to the maximum slope. Smaller U waves (<0.1 mV) should be excluded whereas larger U waves merging with T waves should be included in the measurements [Anderson et al. 2002]. There are no standards for interpreting prolonged QT intervals from Holter or 24/48 h ambulatory monitoring records available. As result, ambulatory monitoring of QT assessment is not recommended.
Several factors such as gender, heart rate, underlying rhythm and conduction defects influence the QT interval. It is also influenced by the physiologic and metabolic state of the patients. Numerous methodologies for correcting QT intervals for heart rate have been proposed, and each has its own benefits and shortcomings. There is no consensus as to which one is the most effective. However, the most universally adopted method is Bazett's formula (QTc = QT/&radix;RR in seconds) that provides an adequate correction for heart rate ranging anywhere between 60 and 100 beats/min. Nonetheless, it underestimates and overestimates the QT interval at low and high heart rates, respectively.
For heart rates outside the normal range, other correction methods such as Fredericia (QTc = QT/(RR)1/3) or Framingham (QTc = QT + 0.154(1 – RR) should be utilized (Table 1) [Aytemir et al.1999]. However, these correction methods are based on population mean correction factor and do not address intra- or inter-individual variability. As there is now a strong evidence for significant inter-individual variability, the best HR correction for QT should be estimated for each individual [Batchvarovet al. 2002; Malik et al. 2002; Couderc et al. 2005].
Estimation of individual correction factor is a cumbersome, time-consuming process. It is preferred in clinical studies but not applicable in clinical practice [Piotrovsky, 2005]. Fossa and colleagues proposed a QT-HR nomogram based on a QT-RR cloud diagram developed from human preclinical studies (Figure 3) [Chan et al. 2007] that can readily be used in the clinical setting. The nomogram incorporates HR rather than RR interval and is found to be safe, with excellent sensitivity, and at the same time is specific enough to allow the assessment of many patients as 'not at risk' for drug-induced TdP and therefore not requiring cardiac monitoring [Chan et al. 2007]. The performance of the nomogram in patients who incurred an antidepressant overdose but did not develop arrhythmia was studied by Bateman and colleagues [Waring et al. 2010].
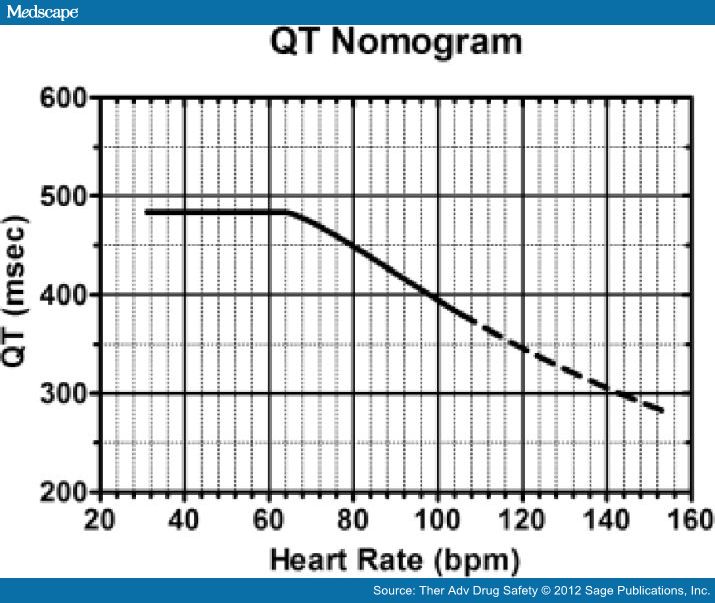
The QT nomogram was associated with a lower false-positive rate than other widely accepted QTc criteria. The greatest discrepancy between the nomogram and QTc methods was amongst patients with heart rates between 30 and 60 beats/min. For example, patients with a citalopram overdose had QT values above the nomogram compared with venlafaxine and mirtazapine overdose, predicting its higher risk for developing arrhythmia and thus the need for close cardiac monitoring [Waring et al.2010].
Based on Bazett's corrected QTc value, in adult males a QT interval greater than 450 ms is considered prolonged and between 430 and 450 ms is considered borderline. For females, a QT interval greater than 470 ms is considered prolonged and between 450 and 470 ms is considered borderline [Goldenberg et al. 2006].
The Mechanism of TdP in the Setting of QT Prolongation
The danger inherent in a prolonged QT is that excessive QT prolongation carries a risk of sudden cardiac death (SCD) due to polymorphic tachycardia, also known as TdP. Prolongation of ventricular repolarization often leads to oscillation in the membrane potential called early after depolarization (EAD). If the EAD reaches a critical threshold in a large area of myocardium, it can result in an ectopic beat [January and Riddle, 1989]. This ectopic beat is usually followed by a long pause with a subsequent sinus beat showing marked QT prolongation. In the presence of an exaggerated heterogeneity of action potential duration across the myocardium, the ectopic beat can induce reentrant excitation and TdP [Nguyen et al.2010] (Figure 4).
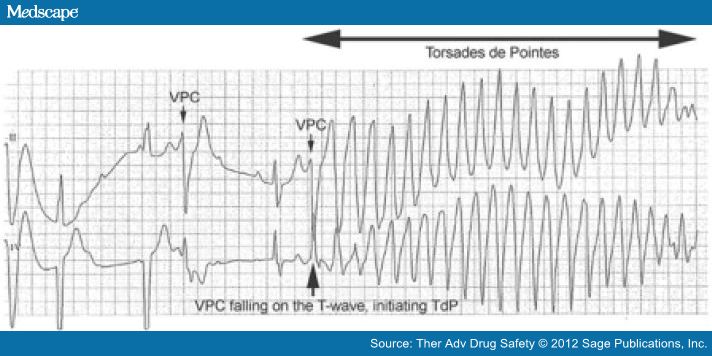
Figure 4.
Ventricular premature contractions (VPCs) occurring in a patient with heart block and prolonged QT. The timing of the second VPC (arrow) is such that it occurs on the T-wave of a preceding T-wave, instigating an episode of Torsades de Pointes (TdP).
This pattern of onset of a short-long-short cycle is typical of drug-induced TdP, sometimes referred as pause-dependant TdP [El-Sherif et al. 1999]. This pattern is in contrast to congenital form, where TdP often follows a sudden adrenergic surge such as exercise or arousal. Irrespective of the mechanism, TdP usually does not sustain long and terminates spontaneously. However, if it happens successively, it can degenerate into ventricular fibrillation and SCD [Passman and Kadish, 2001].
Concomitant Risk Factors Predisposing Patients to LQTS
Drug-induced LQTS is unpredictable in any given individual. The relationship between the action of a drug on a molecular level and the expected clinical effect is not always concordant [Roden, 2004; Yang et al. 2001]. For example, in patients with a normal baseline QT, amiodarone prolongs the QT interval but very rarely causes TdP [Lazzara, 1989]. In contrast, terfenadine, a potent IKr blocker, causes very minimal QT prolongation but was frequently implicated in TdP resulting in its withdrawal from market [Monahan et al. 1990]. So, it appears that certain additional risk factors play a major role in facilitating druginduced TdP (Box 1). In one study, almost all patients who developed TdP had one risk factor and 71% had multiple risk factors [Zeltser et al. 2003].
The most common risk is female sex [Makkar et al. 1993]. After puberty, women have a longer baseline QT interval and respond adversely to IKr blocking drugs as compared with males. Although the mechanism for the gender difference in repolarization is not clearly understood, it has been shown that androgens increase IKs and IKr channels and thereby reduce AP duration [Arya, 2005; Pham and Rosen, 2002]. Heart rate and extracellular potassium concentration also play a significant role. Bradycardia decreases potassium outflow during phase 3 repolarization and thereby increases the QT interval. On the other hand, hypokalemia, potentiates the drug-induced inhibition of IKr channels [Yang and Roden, 1996].
In heart failure and LVH, there is a downregulation of K channels and upregulation of Ca channels [Brooksby et al. 1993; Tomaselli et al. 1994]. This increases the risk for QT prolongation and TdP.
Interaction between concomitant drugs can lead to prolonged QT interval if they have additive or potentiating effect, for example combination of antiarrhythmic agents. Pharmacokinetic interactions may occur if one drug reduces the clearance of the other or if both the drugs compete for the same hepatic enzyme [Cholerton et al. 1992]. Co-administering a drug with cytochrome CYP3A4 inhibitors such as '-azoles', '-mycins' or grapefruit juice will increase its level [Zitron et al. 2005]. Haloperidol and thioridazine share the same enzyme CYP2D6 for clearance resulting in increased levels of both [Aerssens and Paulussen, 2005].
Genetic Predisposition to LQTS
It is known that 5–20% of patients with druginduced TdP have mutations in genes which cause LQTS [Paulussen et al. 2004]. These patients are generally asymptomatic with normal to borderline QTc interval at baseline but become more susceptible to QT prolongation and TdP, when exposed to some drugs [Aerssens and Paulussen, 2005; Paulussen et al.2004].
In addition, polymorphism of the genes coding for the CYP2D6 enzyme can lead to poor metabolism of CYP2D6 dependant drugs. Nearly 5–10% of White patients are considered to be poor metabolizers and are at risk for QT prolongation and TdP especially if the parent drug has the tendency to cause QT prolongation [Bradford, 2002].
In vitro, the degree of hERG blockade and the risk for TdP can be determined by a ratio called EPTC/IC50, also known as therapeutic/toxic ratio (EPTC: effective plasma therapeutic concentration; IC50: in vitro concentration that blocks 50% of hERG). The risk of developing TdP increases as the ratio goes up. Medications such as cisapride, sparfloxacin, quinidine, ibutilide, and thioridazine were found to have a ratio of greater than 1 [De Bruin et al. 2005].
Increased Incidence of LQTS With Specific Drugs
The incidence of drug-induced TdP in general population is largely unknown. It varies depending on the population studied and the type of drugs used. In one observational study, 3.1% of the patients taking noncardiac medications developed TdP. The list of some drugs that can cause QT prolongation is listed in Box 2.
Antiarrhythmic Agents
Antiarrhythmic agents are the leading cause of drug-induced TdP.
Class IA agents (quinidine, procainamide and disopyramide) block both Na and K channels, and TdP can occur either at therapeutic or subtherapeutic doses [Jackman et al. 1988]. Quinidine prolongs QT interval by an average of 10–15% within a week of initiation of therapy and carries a 1.5% risk of inducing TdP [Roden et al. 1986]. TdP while on antiarrhythmic therapy is often precipitated by hypokalemia or hypomagnesaemia. The mortality increases by threefold when used in atrial fibrillation for rhythm maintenance [Coplen et al. 1990]. Procainamide is less likely to induce TdP due to its predominant Na blocking effect. However, it can induce TdP in patients with renal dysfunction or in rapid acetylators through its active metabolite, N-acetyl procainamide (NAPA), that has a potent K blocking effect [Olshansky et al. 1982]. Disopyramide has also been implicated in TdP and is contraindicated in patients with heart failure, liver or renal dysfunction [Lo et al. 1980].
Class III agents are potent IKr blockers and prolong QT interval in a dose-dependent manner. The potassium blocking effect is maximum at low heart rate due to reverse use dependency feature [Hondeghem and Snyders, 1990]. Dofetilide, ibutilide and sotalol carry the highest risk for TdP whereas amiodarone has the lowest risk. Sotalol causes TdP in 2–4% of patients with a higher risk in women [Lehmann et al. 1996]. Dofetilide's incidence of TdP is 2.1% with increased risk in renal failure patients [Torp-Pedersen et al. 1999]. Intravenous ibutilide carries TdP risk of 1–3% [Stambler et al. 1996] with higher incidence in patients with structural heart disease, heart failure and electrolyte disturbance. Amiodarone rarely causes TdP despite its QT prolonging effect. It can be explained by some of its unique features such as lack of reverse use dependency, decreased QT dispersion across ventricular myocardium, L-type calcium blocking effects and B blocking effect.
Antihistamines
Nonsedating antihistamines are widely prescribed but only a few drugs were implicated with significant arrhythmogenicity. Terfenadine and astemizole have been associated with TdP due their potent IKr blocking effect even at lower doses. Both have been withdrawn from market.
Most antihistamines are metabolized by the cytochrome P450 enzyme, CYP3A4. Patients with liver dysfunction or co-administration of drugs or food that inhibit the CYP3A4 can result in higher drug levels. The incidence of pro-arrhythmia with the newer nonsedating antihistamines is unknown but they seem to be safer than previous versions.
Antipsychotic Medications
Antipsychotic medications prolong QT interval in a dose-dependent manner, and are well known to cause TdP.
Haloperidol (a butyrophenone) is widely used to treat schizophrenia and severe agitations. It is a potent blocker of IKr channel and prolongs QT interval by 15–30 ms [Glassman and Bigger, 2001]. The effect is amplified in the presence of risk factors (Box 1). In 2007, the United States Food and Drug Administration (FDA) released an alert suggesting ECG monitoring with its intravenous use. However, based on the available data, it is felt to be safe to give IV haloperidol up to a cumulative dose of 2 mg in patients who do not have risk factors without ECG monitoring [Meyer-Massetti et al. 2010]. Droperidol has a similar effect of haloperidol and careful attention to be paid in patients with risk factors.
Phenothiazines such as chlorpromazine have an antipsychotic and anti-emetic effect. Both chlorpromazine and thioridazine have been implicated with QT prolongation and TdP due to their K blocking effect.
Atypical Antipsychotics
Most of the atypical antipsychotics can cause dose-dependent prolongation of the QT interval with variation in their potency. QT prolongation is highest with ziprasidone and lowest with olanzapine [Vieweg, 2003]. Sertindole was withdrawn from market in 1998 due to the risk of TdP and sudden death. Zimelidine and citalopram have been related to TdP in toxic doses [Liljeqvist and Edvardsson, 1989; Personne et al. 1997].
Antidepressants
Tricyclic antidepressants (TCAs) are more commonly associated with prolongation of the QTc interval than are selective serotonin-reuptake inhibitors (SSRIs) [Ray et al. 2004]. TCAs prolong the QTc predominantly by blocking the Na channel. The effect is more pronounced if a potassium channel blocking agent is co-administered. Amitriptyline, desipramine and imipramine have all been implicated with TdP [Casazza et al. 1986]. Toxic doses of TCA can result in various EKG changes such as widening of QRS complexes, QT prolongation and TdP. SSRIs prolong the QT interval by inhibiting the IKr channel. Citalopram and escitalopram are also associated with QT prolongation.
Antibiotics
Fluoroquinolones have a variable effect on QTc interval with very rare incidence of TdP. Grepafloxacin and sparfloxacin delay repolarization more profoundly than gatifloxacin, levofloxacin, and moxifloxacin, with ciprofloxacin and ofloxacin causing the least effect on the IKr channel [Anderson et al. 2001]. Both grepafloxacin and sparfloxacin were discontinued in the preliminary drug development. The rest of the fluoroquinolones are relatively safe but caution should be applied if there are any underlying risk factors or with co-administration of QT-prolonging drugs [Anderson et al. 2001].
Macrolides such as clarithromycin and erythromycin have been associated with QT prolongation and TdP [Lee et al. 1998; Ray et al. 2004]. In animal studies, erythromycin was found to have similar effects to class III antiarrhythmic agents with prolongation of QT interval, induction of EAD and transmural dispersion. Both erythromycin and clarithromycin are CYP3A4 inhibitors and can lead to significant toxicity if co-administered with another CYP3A4 inhibitor or with drugs that are metabolized by CYP3A4. Although azithromycin is considered safe, TdP has been reported with its use as well [Huang et al.2007].
Antimalarial agents are commonly prescribed worldwide. The true incidence of QT prolongation and TdP with their use is largely unknown due to underreporting. Quinine, an optical isomer of quinidine, has a weak effect on QT interval and has been rarely associated with TdP [Martin et al. 1997].
Chloroquine and halofantrine can prolong the QT interval and have been linked to TdP. Halofantrine is the most potent agent with repolarization properties similar to quinidine and Class III antiarrhythmic agents [Wesche et al. 2000].
Pentamidine is an antiprotozoal drug that is used for Pneumocystis carinii pneumonia treatment. The electrophysiologic properties of pentamidine are unknown but it has a structural similarity to procainamide. Intravenous use can lead to TdP whereas the inhaled form is considered safe [Eisenhauer et al. 1994; Cardoso et al. 1997]. The proarrhythmic risk of IV use is related to idiosyncratic reaction rather than dose effect [Eisenhauer et al. 1994].
Systemic azole group of antifungal agents have both pharmacodynamic and pharmacokinetic characteristics that may trigger TdP [Owens, 2004]. Attention should be paid with its use especially in patients who have underlying risk factors for QT prolongation.
Other Agents
Cisapride is a gastrointestinal promotility agent used for gastroesophageal reflux disease (GERD) and delayed gastric emptying time. It is structurally similar to procainamide and has both IKr and IKs blocking effect (IKr>IKs) [Carlsson et al. 1997]. Cisapride use was related to the greatest number of TdP cases next to antiarrhythmic agents [Wysowski et al. 2001] resulting in its withdrawal from US market in July 2000.
The Screening Method for Long QT in Preclinical Drug Development
In view of the high risk for QT prolongation and TdP with various drugs, it has now become a common practice for the pharmaceutical companies and biotechnology companies to screen compounds for hERG channel activity early during preclinical safety assessment.
A centerpiece of this practice was the establishment of the 'thorough QT/QTc study' that was intended to confidently identify drugs that may cause QT prolongation. This study has evolved as a key component of all clinical development programs for new molecular entities. It is a doubleblinded, randomized design with a placebo and positive control arm that is strictly powered to exclude an effect on the QTc interval exceeding 10 ms. It is conducted in healthy volunteers once the tolerability and the pharmacokinetics of the drug have been established. During the studies, RR interval, heart rate, QT interval corrected using both Bazetts's (QTcB = QT/RR0.5) and Fridericia's correction (QTcF = QT/RR0.33) are collected. ECG interval measurement methods are either 'fully manual' or use some degree of 'manual adjudication' (measurements made by a computer-based algorithm are reviewed and corrected as needed) [FDA, 2005].
The role of the positive control is to demonstrate the study's ability (i.e. 'assay sensitivity') to detect a small effect on the QT interval; in this case QT prolongation slightly over 5 ms. If the study is able to detect such a small QT prolongation by the control, then a finding of a lesser QT effect for the test drug should indicate that the test drug does not significantly prolong the QT interval. In the vast majority of TQT studies, moxifloxacin, a fluoroquinoline antibiotic with a mild QT prolonging effect has been used. Moxifloxacin 400 mg was associated with an observed 7.5– 12.5 ms increase in the mean placebo- and baseline- corrected QTc interval that supports the appropriateness of its use as a positive control in TQT studies [Bloomfield et al. 2008].
A QT study usually results in three clinical scenarios: the studied drug prolongs the mean QTc interval by ≤10 ms and therefore does not appear to cause TdP (or the increased risk is too small to be detected). The studied drug prolongs the mean QTc interval by >10 ms but ≤20 ms, and so has an 'uncertain' risk of inducing TdP. The studied drug prolongs the mean QT/QTc interval by >20 ms, and may have a high risk for causing clinically important arrhythmic events. A 'negative' result would most likely allow standard ECG data to be collected in accordance with standard practice in phase II–III of clinical drug testing. A 'nonnegative' result (QTc interval >10 ms) requires comprehensive dose–response data to be obtained through the expansion of ECG data collection in phase II and III trials.
In order to fully evaluate the risk of QT prolongation, it is important to include patients in these analyses with additional risk factors for TdP such as patients with electrolyte abnormalities (e.g. hypokalemia), congestive heart failure, impaired drug metabolizing capacity or clearance (e.g. renal or hepatic impairment, drug interactions), female patients and patients aged <16 and over 65 years.
Recent studies have indicated that fully or partially automated methods of QT interval measurements using computer algorithms, compared with the labor-intensive manual methods, produce similar results when tested on drugs with a known QT-prolonging effect [Fosser et al. 2009; Sarapa et al. 2009]. It is expected that use of automated QT algorithms in the evaluation of new medications will not only to replace manual QT measurements but also will improve the detection of subtle T-wave changes [Darpo, 2010].
The Treatment of TdP
The treatment depends on the hemodynamic stability of the patient. For patients with TdP that does not terminate spontaneously or that degenerates into ventricular fibrillation, immediate direct-current cardioversion should be performed. Those who are stable can be treated in the following ways:
-
Intravenous magnesium is the first line of choice even in patients with normal magnesium levels. It is effective acutely and also prevents future occurrence of ectopic beats and TdP. It works by decreasing the influx of calcium current and thereby lowering the amplitude of EAD which ultimately prevents ectopic beats. The dose is 2 g over 1–2 min followed by an infusion of 2–4 mg/min. Repeat bolus of 2 g should be given for recurrence [Banai and Tzivoni, 1993].
-
Intravenous potassium should be considered even in normokalemic patients (0.5 meq/kg to a mean of 40 meq) as it has shown to suppress QT abnormalities in the acute phase [Choy et al.1997].
-
When a patient does not respond to IV magnesium, overdrive transvenous pacing should be considered with a target heart rate of between 90 and 110 beats/min. This shortens the QT interval, and decreases EAD and QT dispersion [Khan, 2002].
-
Isoproterenol can be used as a bridge to temporary pacemaker or if a pacemaker is not available to increase heart rate. However, it is contraindicated in patients with congenital LQTS.
-
The patient's medication list should be reviewed in detail with regards to potential culprit drugs. The website www.qtdrugs.org provides an excellent up-to-date reference on QT-prolonging drugs.
-
Permanent pacemakers should be inserted in patients who have chronic bradycardia such as sick sinus syndrome or AV block. Implantable cardioverter–defibrillators (ICDs) are indicated in cases that cannot be managed by avoidance of the offending agent.
Prevention and Monitoring of Druginduced QT Prolongation
QT-prolonging drugs should be avoided in patients with pre-existing heart disease, history of ventricular arrhythmias or with metabolic abnormalities such as hypokalemia. Hospitalized patients come under high risk for developing TdP than outpatients with the same QT prolonging drugs. Hospitalized patients are often elderly people with underlying heart disease who may also have renal or hepatic dysfunction, electrolyte abnormalities, or bradycardia and to whom drugs may be administered rapidly via the intravenous route [Drew et al.2010]. Concomitant administration of drugs that inhibit the cytochrome P450 especially imidazole antifungals, macrolide antibiotics or those that can prolong the QT interval or drugs that cause electrolyte disturbance should be avoided. It is recommended to perform surveillance EKGs before and after initiation of QT-prolonging drugs. Routine monitoring of electrolytes especially potassium is also recommended in those who are on diuretics and QT-prolonging drugs.
For example, methadone has been associated with TdP in women if the dose is >100 mg/day [Stringer et al. 2009]. Nearly, one million Americans take methadone either for chronic pain or narcotic dependency [Krantz et al. 2009]. Methadone guidelines recommend a pretreatment ECG for QTc interval screening and a follow-up ECG within 30 days and then annually [Krantz et al. 2009].
EKG monitoring of the QT interval in the hospital setting is indicated for the following reasons: (1) initiation of a drug known to cause TdP; (2) overdose from potentially proarrhythmic agents; (3) new-onset bradyarrhythmias; and (4) severe hypokalemia or hypomagnesemia [Drew et al. 2004].
If drug-induced TdP has occurred, a careful review of the patient's personal and family history should be obtained in order to identify the possibility of a congenital LQTS. If a personal/family history of unexplained syncope or premature sudden death is discovered, a 12-lead ECG should be recommended for all first-degree relatives and consideration should be given to clinically available genetic testing for congenital LQTS.
Conclusion
Drug-induced QT prolongation and TdP are more prevalent than previously thought. Accurate calculation of baseline QT interval, careful review of a patient's medication list, and obtaining a thorough family history are paramount to avoiding iatrogenic QT prolongation. When TdP occurs it is imperative to identify the potential offending agent, discontinue it, and to take steps to treat the life-threatening rhythm disturbances.
References
-
Aerssens, J. and Paulussen, A. (2005) Pharmacogenomics and acquired long QT syndrome. Pharmacogenomics 6: 259–270.
-
Anderson, M., Al-Khatib, S., Roden, D. and Califf, R. (2002) Cardiac repolarization: current knowledge, critical gaps, and new approaches to drug development and patient management. Am Heart J 144: 769–781.
-
Anderson, M., Mazur, A., Yang, T. and Roden, D. (2001) Potassium current antagonist properties and proarrhythmic consequences of quinolone antibiotics. J Pharmacol Exp Ther 296: 806–810.
-
Arya, A. (2005) Gender-related differences in ventricular repolarization: beyond gonadal steroids. J Cardiovasc Electrophysiol 16: 525–527.
-
Aytemir, K., Maarouf, N., Gallagher, M., Yap, Y., Waktare, J. and Malik, M. (1999) Comparison of formulae for heart rate correction of QT interval in exercise electrocardiograms. Pacing Clin Electrophysiol 22: 1397–1401.
-
Banai, S. and Tzivoni, D. (1993) Drug therapy for Torsade de Pointes. J Cardiovasc Electrophysiol 4: 206–210.
-
Batchvarov, V., Ghuran, A., Smetana, P., Hnatkova, K., Harries, M., Dilaveris, P. et al. (2002) QT-RR relationship in healthy subjects exhibits substantial intersubject variability and high intrasubject stability. Am J Physiol Heart Circ Physiol 282: H2356–H2363.
-
Berthet, M., Denjoy, I., Donger, C., Demay, L., Hammoude, H., Klug, D. et al. (1999) C-terminal HERG mutations: the role of hypokalemia and a KCNQ1-associated mutation in cardiac event occurrence. Circulation 99: 1464–1470.
-
Bloomfield, D., Kost, J., Ghosh, K., Hreniuk, D., Hickey, L., Guitierrez, M. et al. (2008) The effect of moxifloxacin on QTc and implications for the design of thorough QT studies. Clin Pharmacol Ther 84: 475–480.
-
Bradford, L. (2002) CYP2D6 allele frequency in European Caucasians, Asians, Africans and their descendants.Pharmacogenomics 3: 229–243.
-
Brooksby, P., Levi, A. and Jones, J. (1993) The electrophysiological characteristics of hypertrophied ventricular myocytes from the spontaneously hypertensive rat. J Hypertens 11: 611–622.
-
Cardoso, J., Mota-Miranda, A., Conde, C., Moura, B., Rocha-Goncalves, F. and Lecour, H. (1997) Inhalatory pentamidine therapy and the duration of the QT interval in HIV-infected patients. Int J Cardiol 59: 285–289.
-
Carlsson, L., Amos, G., Andersson, B., Drews, L., Duker, G. and Wadstedt, G. (1997) Electrophysiological characterization of the prokinetic agents cisapride and mosapride in vivo and in vitro: implications for proarrhythmic potential? J Pharmacol Exp Ther 282: 220–227.
-
Casazza, F., Fiorista, F., Rustici, A. and Brambilla, G. (1986) [Torsade de pointes caused by tricyclic antidepressive agents. Description of a clinical case]. G Ital Cardiol 16: 1058–1061.
-
Chan, A., Isbister, G., Kirkpatrick, C. and Dufful, S. (2007) Drug-induced QT prolongation and torsades de pointes: evaluation of a QT nomogram. QJM 100: 609–615.
-
Ching, C. and Tan, E. (2006) Congenital long QT syndromes: clinical features, molecular genetics and genetic testing. Expert Rev Mol Diagn 6: 365–374.
-
Cholerton, S., Daly, A. and Idle, J. (1992) The role of individual human cytochromes P450 in drug metabolism and clinical response. Trends Pharmacol Sci 13: 434–439.
-
Choy, A., Darbar, D., Dell'Orto, S. and Roden, D.M. (1999) Exaggerated QT prolongation after cardioversion of atrial fibrillation. J Am Coll Cardiol 34: 396–401.
-
Choy, A., Lang, C., Chomsky, D., Rayos, G., Wilson, J. and Roden, D. (1997) Normalization of acquired QT prolongation in humans by intravenous potassium. Circulation 96: 2149–2154.
-
Coplen, S., Antman, E., Berlin, J., Hewitt, P. and Chalmers, T. (1990) Efficacy and safety of quinidine therapy for maintenance of sinus rhythm after cardioversion. A meta-analysis of randomized control trials. Circulation 82: 1106–1116.
-
Couderc, J., Xiaojuan, X., Zareba, W. and Moss, A. (2005) Assessment of the stability of the individualbased correction of QT interval for heart rate. Ann Noninvasive Electrocardiol 10: 25–34.
-
Darpo, B. (2010) The thorough QT/QTc study 4 years after the implementation of the ICH E14 guidance. Br J Pharmacol 159: 49–57.
-
De Bruin, M., Pettersson, M., Meyboom, R., Hoes, A. and Leufkens, H. (2005) Anti-HERG activity and the risk of drug-induced arrhythmias and sudden death. Eur Heart J 26: 590–597.
-
Drew, B., Ackerman, M., Funk, M., Gibler, W., Kligfield, P., Menon, V. et al. (2010) Prevention of Torsade de Pointes in hospital settings: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. Circulation 121: 1047–1060.
-
Drew, B., Califf, R., Funk, M., Kaufman, E., Krucoff, M., Laks, M. et al. (2004) Practice standards for electrocardiographic monitoring in hospital settings: an American Heart Association scientific statement from the Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young: endorsed by the International Society of Computerized Electrocardiology and the American Association of Critical-Care Nurses.Circulation 110: 2721–2746.
-
Eisenhauer, M., Eliasson, A., Taylor, A., Coyne, P., Jr, and Wortham, D. (1994) Incidence of cardiac arrhythmias during intravenous pentamidine therapy in HIV-infected patients. Chest 105: 389–395.
-
El-Sherif, N., Caref, E, Chinushi, M. and Restivo, M. (1999) Mechanism of arrhythmogenicity of the short-long cardiac sequence that precedes ventricular tachyarrhythmias in the long QT syndrome. J Am Coll Cardiol 33: 1415–1423.
-
FDA. (2005) International Conference on Harmonisation; guidance on E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs; availability. Notice. Fed Regist 70: 61134–61135.
-
Ficker, E., Kuryshev, Y., Dennis, A., Obejero-Paz, C., Wang, L., Hawryluk, P. et al. (2004) Mechanisms of arsenic-induced prolongation of cardiac repolarization. Mol Pharmacol 66: 33–44.
-
Fossa, A., Wisialowski, T., Magnano, A., Wolfgang, E., Winslow, R., Gorczyca, W. et al. (2005) Dynamic beatto- beat modeling of the QT-RR interval relationship: analysis of QT prolongation during alterations of autonomic state versus human ether a-go-go-related gene inhibition. J Pharmacol Exp Ther 312: 1–11.
-
Fosser, C., Duczynski, G., Agin, M., Wicker, P. and Darpo, B. (2009) Comparison of manual and automated measurements of the QT interval in healthy volunteers: an analysis of five thorough QT studies. Clin Pharmacol Ther 86: 503–506.
-
Glassman, A. and Bigger, J., Jr, (2001) Antipsychotic drugs: prolonged QTc interval, Torsade de Pointes, and sudden death. Am J Psychiatry 158: 1774–1782.
-
Goldenberg, I., Moss, A. and Zareba, W. (2006) QT interval: how to measure it and what is "normal". J Cardiovasc Electrophysiol 17: 333–336.
-
Hedley, P., Jorgensen, P., Schlamowitz, S., Wangari, R., Moolman-Smook, J., Brink, P. et al. (2009) The genetic basis of long QT and short QT syndromes: a mutation update. Hum Mutat 30: 1486–1511.
-
Hondeghem, L. and Snyders, D. (1990) Class III antiarrhythmic agents have a lot of potential but a long way to go. Reduced effectiveness and dangers of reverse use dependence. Circulation 81: 686–690.
-
Huang, B., Wu, C., Hsia, C. and Yin Chen, C. (2007) Azithromycin-induced torsade de pointes. Pacing Clin Electrophysiol 30: 1579–1582.
-
Jackman, W., Friday, K., Anderson, J., Aliot, E., Clark, M. and Lazzara, R. (1988) The long QT syndromes: a critical review, new clinical observations and a unifying hypothesis. Prog Cardiovasc Dis 31: 115–172.
-
January, C. and Riddle, J. (1989) Early after depolarizations: mechanism of induction and block. A role for L-type Ca2+ current. Circ Res 64: 977–990.
-
Kannankeril, P. (2008) Understanding drug-induced torsades de pointes: a genetic stance. Expert Opin Drug Saf 7: 231–239.
-
Khan, I. (2002) Clinical and therapeutic aspects of congenital and acquired long QT syndrome. Am J Med 112: 58–66.
-
Krantz, M., Martin, J., Stimmel, B., Mehta, D. and Haigney, M. (2009) QTc interval screening in methadone treatment. Ann Intern Med 150: 387–395.
-
Kuryshev, Y., Ficker, E., Wang, L., Hawryluk, P., Dennis, A., Wible, B. et al. (2005) Pentamidineinduced long QT syndrome and block of hERG trafficking. J Pharmacol Exp Ther 312: 316–323.
-
Kuryshev, Y., Wang, L., Wible, B., Wan, X. and Ficker, E. (2006) Antimony-based antileishmanial compounds prolong the cardiac action potential by an increase in cardiac calcium currents. Mol Pharmacol 69: 1216–1225.
-
Lazzara, R. (1989) Amiodarone and Torsade de Pointes. Ann Intern Med 111: 549–551.
-
Lee, K., Jim, M., Tang, S. and Tai, Y. (1998) QT prolongation and Torsades de Pointes associated with clarithromycin. Am J Med 104: 395–396.
-
Lehmann, M., Hardy, S., Archibald, D., Quart, B. and MacNeil, D. (1996) Sex difference in risk of Torsade de Pointes with d,l-sotalol. Circulation 94: 2535–2541.
-
Liljeqvist, J. and Edvardsson, N. (1989) Torsade de Pointes tachycardias induced by overdosage of zimeldine. J Cardiovasc Pharmacol 14: 666–670.
-
Lo, K., Gantz, K., Stetson, P., Lucchesi, B. and Pitt, B. (1980) Disopyramide-induced ventricular tachycardia. Arch Intern Med 140: 413–414.
-
Makkar, R., Fromm, B., Steinman, R., Meissner, M. and Lehmann, M. (1993) Female gender as a risk factor for torsades de pointes associated with cardiovascular drugs. JAMA 270: 2590–2597.
-
Malik, M., Farbom, P., Batchvarov, V., Hnatkova, K. and Camm, A. (2002) Relation between QT and RR intervals is highly individual among healthy subjects: implications for heart rate correction of the QT interval. Heart 87: 220–228.
-
Martin, E., Rogalski, K. and Black, J. (1997) Quinine may trigger Torsades de Pointes during astemizole therapy.Pacing Clin Electrophysiol 20: 2024–2025.
-
Meyer-Massetti, C., Cheng, C., Sharpe, B., Meier, C. and Guglielmo, B. (2010) The FDA extended warning for intravenous haloperidol and torsades de pointes: how should institutions respond? J Hosp Med 5(4): E8–E16.
-
Modell, S. and Lehmann, M. (2006) The long QT syndrome family of cardiac ion channelopathies: a HuGE review.Genet Med 8: 143–155.
-
Monahan, B., Ferguson, C., Killeavy, E., Lloyd, B., Troy, J. and Cantilena, L., Jr, (1990) Torsades de pointes occurring in association with terfenadine use. JAMA 264: 2788–2790.
-
Monnig, G., Eckardt, L., Wedekind, H., Haverkamp, W., Gerss, J., Milberg, P. et al. (2006) Electrocardiographic risk stratification in families with congenital long QT syndrome. Eur Heart J 27: 2074–2080.
-
Moss, A., Zareba, W., Kaufman, E., Gartman, E., Peterson, D., Benhorin, J. et al. (2002) Increased risk of arrhythmic events in long-QT syndrome with mutations in the pore region of the human ether-a-gogo-related gene potassium channel. Circulation 105: 794–799.
-
Nguyen, A., Sarmast, S., Kowal, R. and Schussler, J. (2010) Cardiac arrest due to torsades de pointes in a patient with complete heart block: the "R-on-T" phenomenon. Proc (Bayl Univ Med Cent) 23: 361–362.
-
Olshansky, B., Martins, J. and Hunt, S. (1982) N-acetyl procainamide causing torsades de pointes. Am J Cardiol50: 1439–1441.
-
Owens, R., Jr, (2004) QT prolongation with antimicrobial agents: understanding the significance. Drugs 64: 1091–1124.
-
Passman, R. and Kadish, A. (2001) Polymorphic ventricular tachycardia, long Q-T syndrome, and torsades de pointes. Med Clin North Am 85: 321–341.
-
Paulussen, A., Gilissen, R., Armstrong, M., Doevendans, P., Verhasselt, P., Smeets, H. et al. (2004) Genetic variations of KCNQ1, KCNH2, SCN5A, KCNE1, and KCNE2 in drug-induced long QT syndrome patients. J Mol Med (Berl) 82: 182–188.
-
Perrin, M., Subbiah, R., Vandenberg, J. and Hill, A. (2008) Human ether-a-go-go related gene (hERG) K+ channels: function and dysfunction. Prog Biophys Mol Biol 98: 137–148.
-
Perry, M., Sanguinetti, M. and Mitcheson, J. (2010) Revealing the structural basis of action of hERG potassium channel activators and blockers. J Physiol 588: 3157–3167.
-
Personne, M., Sjoberg, G. and Persson, H. (1997) Citalopram overdose - review of cases treated in Swedish hospitals. J Toxicol Clin Toxicol 35: 237–240.
-
Pham, T. and Rosen, M. (2002) Sex, hormones, and repolarization. Cardiovasc Res 53: 740–751.
-
Piotrovsky, V. (2005) Pharmacokineticpharmacodynamic modeling in the data analysis and interpretation of drug-induced QT/QTc prolongation. AAPS J 7(3): E609–E624.
-
Rajamani, S., Eckhardt, L., Valdivia, C., Klemens, C., Gillman, B., Anderson, C. et al. (2006) Drug-induced long QT syndrome: hERG K+ channel block and disruption of protein trafficking by fluoxetine and norfluoxetine. Br J Pharmacol 149: 481–489.
-
Ray, W., Murray, K., Meredith, S., Narasimhulu, S., Hall, K. and Stein, C. (2004) Oral erythromycin and the risk of sudden death from cardiac causes. N Engl J Med 351: 1089–1096.
-
Roden, D. (2004) Drug-induced prolongation of the QT interval. N Engl J Med 350: 1013–1022.
-
Roden, D. (2008) Clinical practice. Long-QT syndrome. N Engl J Med 358: 169–176.
-
Roden, D. and Viswanathan, P. (2005) Genetics of acquired long QT syndrome. J Clin Invest 115: 2025–2032.
-
Roden, D., Woosley, R. and Primm, R. (1986) Incidence and clinical features of the quinidineassociated long QT syndrome: implications for patient care. Am Heart J 111: 1088–1093.
-
Sanguinetti, M. and Tristani-Firouzi, M. (2006) hERG potassium channels and cardiac arrhythmia. Nature 440: 463–469.
-
Sarapa, N., Gussak, I., Vajdic, B., George, S., Hadzievski, L., Francom, S. et al. (2009) Comparison of QTinno, a fully automated electrocardiographic analysis program, to semiautomated electrocardiographic analysis methods in a drug safety study in healthy subjects. J Electrocardiol 42: 358–366.
-
Schwartz, P., Priori, S., Spazzolini, C., Moss, A., Vincent, G., Napolitano, C. et al. (2001) Genotypephenotype correlation in the long-QT syndrome: gene-specific triggers for life-threatening arrhythmias. Circulation 103: 89–95.
-
Splawski, I., Shen, J., Timothy, K., Lehmann, M., Priori, S., Robinson, J. et al. (2000) Spectrum of mutations in long-QT syndrome genes. KVLQT1, HERG, SCN5A, KCNE1, and KCNE2. Circulation 102: 1178–1185.
-
Stambler, B., Wood, M., Ellenbogen, K., Perry, K., Wakefield, L. and VanderLugt, J. (1996) Efficacy and safety of repeated intravenous doses of ibutilide for rapid conversion of atrial flutter or fibrillation. Ibutilide Repeat Dose Study Investigators. Circulation 94: 1613–1621.
-
Stringer, J., Welsh, C. and Tommasello, A. (2009) Methadone-associated Q-T interval prolongation and torsades de pointes. Am J Health Syst Pharm 66: 825–833.
-
Swartz, K. (2004) Towards a structural view of gating in potassium channels. Nat Rev Neurosci 5: 905–916.
-
Titier, K., Girodet, P. O.,Verdoux, H., Molimard, M., Begaud, B., Haverkamp, W., Lader, M., Moore, N. (2005) Atypical antipsychotics: from potassium channels to torsade de pointes and sudden death. Drug Saf 28(1): 35–51.
-
Tomaselli, G., Beuckelmann, D., Calkins, H., Berger, R., Kessler, P., Lawrence, J. et al. (1994) Sudden cardiac death in heart failure. The role of abnormal repolarization. Circulation 90: 2534–2539.
-
Torp-Pedersen, C., Moller, M., Bloch-Thomsen, P., Kober, L., Sandoe, E., Egstrup, K. et al. (1999) Dofetilide in patients with congestive heart failure and left ventricular dysfunction. Danish Investigations of Arrhythmia and Mortality on Dofetilide Study Group. N Engl J Med 341: 857–865.
-
Vieweg, W. (2003) New generation antipsychotic drugs and QTc interval prolongation. Prim Care Companion J Clin Psychiatry 5: 205–215.
-
Waring, W., Graham, A., Gray, J., Wilson, A., Howell, C. and Bateman, D. (2010) Evaluation of a QT nomogram for risk assessment after antidepressant overdose. Br J Clin Pharmacol 70: 881–885.
-
Wesche, D., Schuster, B., Wang, W. and Woosley, R. (2000) Mechanism of cardiotoxicity of halofantrine. Clin Pharmacol Ther 67: 521–529.
-
Wysowski, D., Corken, A., Gallo-Torres, H., Talarico, L. and Rodriguez, E. (2001) Postmarketing reports of QT prolongation and ventricular arrhythmia in association with cisapride and Food and Drug Administration regulatory actions. Am J Gastroenterol 96: 1698–1703.
-
Yang, T. and Roden, D.M. (1996) Extracellular potassium modulation of drug block of IKr. Implications for torsade de pointes and reverse use-dependence. Circulation 93: 407–411.
-
Yang, T., Snyders, D. and Roden, D. (2001) Drug block of I(kr): model systems and relevance to human arrhythmias. J Cardiovasc Pharmacol 38: 737–744.
-
Zeltser, D., Justo, D., Halkin, A., Prokhorov, V., Heller, K. and Viskin, S. (2003) Torsade de Pointes due to noncardiac drugs: most patients have easily identifiable risk factors. Medicine (Baltimore) 82: 282–290.
-
Zitron, E., Scholz, E., Owen, R., Luck, S., Kiesecker, C., Thomas, D. et al. (2005) QTc prolongation by grapefruit juice and its potential pharmacological basis: HERG channel blockade by flavonoids. Circulation 111: 835–838.





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 