N Engl J Med 2008; 358:1483-1494April 3, 2008DOI: 10.1056/NEJMra074081
Atopic dermatitis, or eczema, is a common skin disease that is often associated with other atopic disorders, such as allergic rhinitis and asthma.1 The clinical manifestations of atopic dermatitis (FIGURE 1
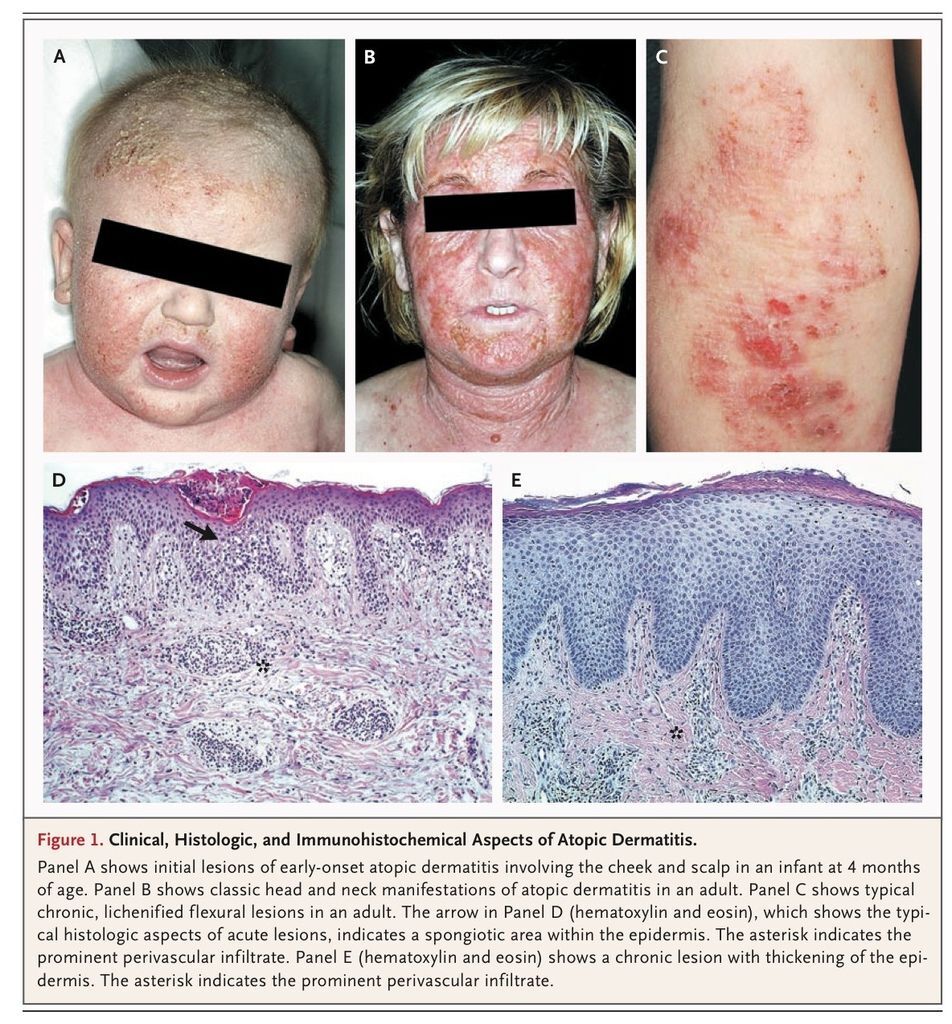
Clinical, Histologic, and Immunohistochemical Aspects of Atopic Dermatitis.) vary with age; three stages can often be identified. In infancy, the first eczematous lesions usually emerge on the cheeks and the scalp. Scratching, which frequently starts a few weeks later, causes crusted erosions. During childhood, lesions involve flexures, the nape, and the dorsal aspects of the limbs. In adolescence and adulthood, lichenified plaques affect the flexures, head, and neck. In each stage, itching that continues throughout the day and worsens at night causes sleep loss and substantially impairs the patient's quality of life.
The hallmarks of atopic dermatitis are a chronic, relapsing form of skin inflammation, a disturbance of epidermal-barrier function that culminates in dry skin, and IgE-mediated sensitization to food and environmental allergens.2 The histologic features of acute eczematous patches and plaques are epidermal intercellular edema (spongiosis) and a prominent perivascular infiltrate of lymphocytes, monocyte macrophages, dendritic cells, and a few eosinophils in the dermis. In subacute and chronic lichenified and excoriated plaques, the epidermis is thickened and its upper layer is hypertrophied.
Two hypotheses concerning the mechanism of atopic dermatitis have been proposed. One holds that the primary defect resides in an immunologic disturbance that causes IgE-mediated sensitization, with epithelial-barrier dysfunction regarded as a consequence of the local inflammation. The other proposes that an intrinsic defect in the epithelial cells leads to the barrier dysfunction; the immunologic aspects are considered to be an epiphenomenon.
In this review, I arrange the disparate pieces of the puzzle into a coherent picture, question prevailing hypotheses, and integrate the results of recent research in a way that has implications for the clinical management of atopic dermatitis.
EPIDEMIOLOGY OF ATOPIC DERMATITIS
The prevalence of atopic dermatitis has doubled or tripled in industrialized countries during the past three decades; 15 to 30% of children and 2 to 10% of adults are affected.3 This disorder is often the prelude to an atopic diathesis that includes asthma and other allergic diseases. Atopic dermatitis frequently starts in early infancy (so-called early-onset atopic dermatitis). A total of 45% of all cases of atopic dermatitis begin within the first 6 months of life, 60% begin during the first year, and 85% begin before 5 years of age. More than 50% of children who are affected in the first 2 years of life do not have any sign of IgE sensitization, but they become sensitized during the course of atopic dermatitis.4 Up to 70% of these children have a spontaneous remission before adolescence. The disease can also start in adults (so-called late-onset atopic dermatitis), and in a substantial number of these patients there is no sign of IgE-mediated sensitization.5 The lower prevalence of atopic dermatitis in rural as compared with urban areas suggests a link to the “hygiene hypothesis,” which postulates that the absence of early childhood exposure to infectious agents increases susceptibility to allergic diseases.6 This concept has recently been questioned with regard to atopic dermatitis, however.3,7
GENETICS OF ATOPIC DERMATITIS
The concordance rate for atopic dermatitis is higher among monozygotic twins (77%) than among dizygotic twins (15%).8 Allergic asthma or allergic rhinitis in a parent appears to be a minor factor in the development of atopic dermatitis in the offspring, suggesting atopic dermatitis–specific genes.9
Genomewide scans10 have highlighted several possible atopic dermatitis–related loci on chromosomes 3q21,11 1q21, 16q, 17q25, 20p,12 and 3p26.13 The region of highest linkage was identified on chromosome 1q21, which harbors a family of epithelium-related genes called the epidermal differentiation complex.14 Most of the genetic regions associated with atopic dermatitis correspond to loci associated with psoriasis, although these two diseases are rarely linked. Also, the genomic associations revealed by these scans do not overlap with allelic variants that are frequent in allergic asthma15; this finding is consistent with epidemiologic data.3,4,16
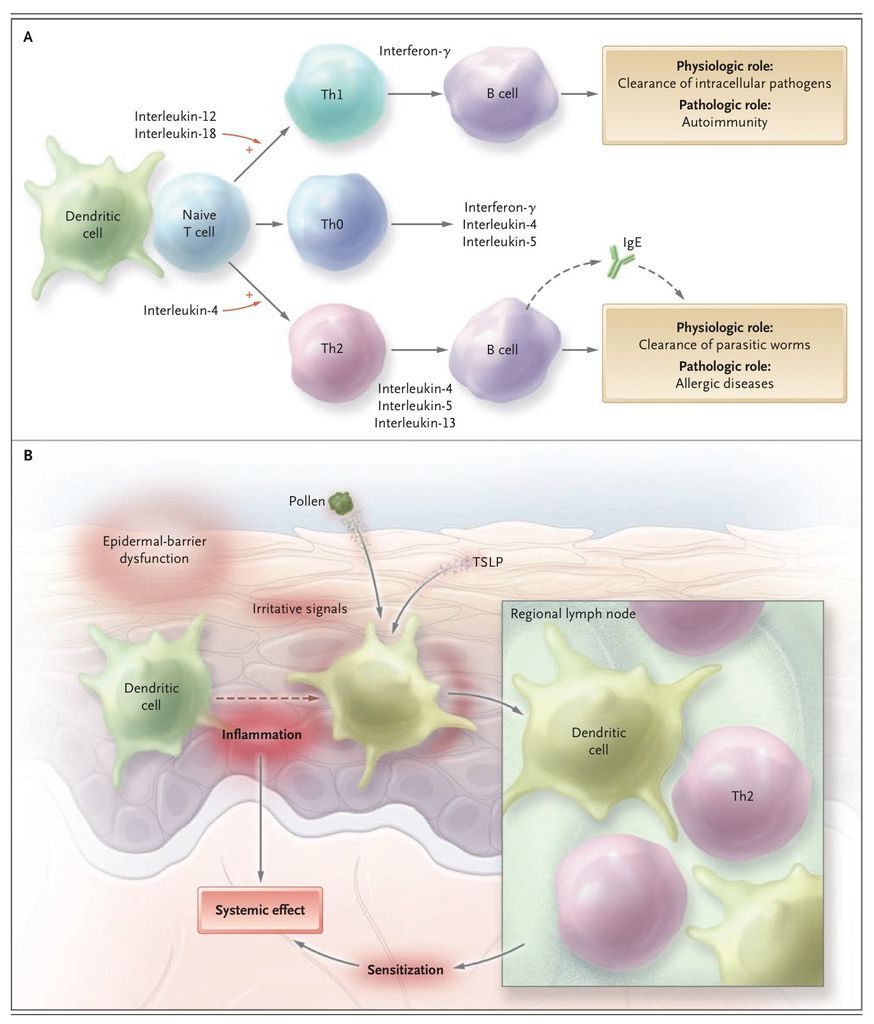
Several candidate genes have been identified in atopic dermatitis,9,17 notably on chromosome 5q31-33. All of them encode cytokines involved in the regulation of IgE synthesis: interleukin-4, interleukin-5, interleukin-12, interleukin-13, and granulocyte–macrophage colony-stimulating factor (GM-CSF). These and other cytokines are produced by two main types of T lymphocytes. Type 2 helper T cells (Th2) produce interleukin-4 as well as interleukin-5 and interleukin-13, two cytokines that up-regulate the production of IgE. Type 1 helper T cells (Th1) produce mainly interleukin-12 and interferon-γ, which suppresses production of IgE and stimulates production of IgG antibodies (FIGURE 2
The Th1–Th2 Paradigm and Its Role in Allergy and the Skin as the Site of Initiation for Sensitization.). Mutations that affect the function of the promoter region of the lymphocyte-attracting chemokine RANTES (regulated on activation, normal T-cell expressed and secreted) (17q11) and gain-of-function polymorphisms in the α subunit of the interleukin-4 receptor (16q12) have been identified in patients with atopic dermatitis. Polymorphisms of the gene encoding the cytokine interleukin-18,18 which contributes to the shift of Th1 and Th2 cross-regulation toward Th1-mediated responses (so-called Th1 polarization), or polymorphisms of the genes encoding receptors of the innate immune system19,20 may contribute to the imbalance between Th1 and Th2 immune responses in atopic dermatitis. In persons with atopic dermatitis, a genetically determined dominance of Th2 cytokines affects the maturation of B cells and a genomic rearrangement in these cells that favors isotype class switching from IgM to IgE.
Since dry and scaly skin is a symptom of both atopic dermatitis and ichthyosis vulgaris, the most common autosomal dominant disorder of keratinization, both diseases might overlap genetically. After the filaggrin gene (FLG) on chromosome 1q21.3, which encodes a key protein in epidermal differentiation, was identified as the gene involved in ichthyosis vulgaris,21 several loss-of-function mutations of the gene were identified in European patients with atopic dermatitis,22-25 and other, distinctive FLG mutations in Japanese patients have been reported.25,26 Mutations of FLG occur mainly in early-onset atopic dermatitis and indicate a propensity toward asthma. There is, however, no association between mutant FLG and allergic airway diseases without atopic dermatitis. SinceFLG mutations are identified in only 30% of European patients with atopic dermatitis, genetic variants of other epidermal structures, such as the stratum corneum tryptic enzyme or a new epidermal collagen, may be important.27,28
Atopic dermatitis is a complex genetic disease that arises from gene–gene and gene–environment interactions. The disease emerges in the context of two major groups of genes: genes encoding epidermal or other epithelial structural proteins, and genes encoding major elements of the immune system.
BARRIER FUNCTION OF THE SKIN
Physical Barrier
An intact epidermal compartment is a prerequisite for the skin to function as a physical and chemical barrier. The barrier itself is the stratum corneum, the brick and mortar–like structure of the upper epidermal layer.29 An alteration of the barrier that causes increased transepidermal water loss is a hallmark of atopic dermatitis. Intercellular lipids of the epidermal horny layers are provided by lamellar bodies, which are produced by exocytosis from upper keratinocytes. Changes in skin ceramides that are secondary to variations in the pH of the stratum corneum can disturb maturation of lamellar bodies and impair the barrier. 30 Alterations in the expression of enzymes involved in the subtle balance of epidermal adhesion structures are also likely to contribute to the breakdown of the epidermal barrier in patients with atopic dermatitis.27,31
Whether these epidermal alterations are primary or are secondary to the underlying inflammation remained unclear until immunohistochemical32 and genetic studies highlighted the importance ofFLG mutations in atopic dermatitis. FLG contributes to the keratin cytoskeleton by acting as the template for the assembly of the cornified envelope; moreover, breakdown products of FLGcontribute to the water-binding capacity of the stratum corneum.33 Genetic variants of FLG in atopic dermatitis that lack the capacity to be proteolytically cleaved have been identified,22 but other genetically determined alterations of the epidermis (e.g., changes in the cornified envelope proteins involucrin and loricrin) or lipid composition are also likely to contribute to barrier dysfunction.14 Underlying inflammation can alter the expression of genes such as FLG that are involved in epidermal-barrier function,34 allowing increased transepidermal penetration of environmental allergens35,36 and, in collaboration with pruritus, fostering inflammation and sensitization.36,37
The Innate Immune System
Epithelial cells at the interface between the skin and the environment are the first line of defense of the innate immune system.38 They are equipped with a variety of sensing structures, which include the toll-like receptors (TLRs),39 C-type lectins, nucleotide-binding oligomerization domain–like receptors, and peptidoglycan-recognition proteins.40 At least 10 different TLRs have been described in humans; they bind to bacterial, fungal (both cell walls), or viral structures (DNA or RNA with so-called cytosine phosphate guanidine [CpG] motifs), and to other microbial structures termed the pathogen-associated molecular patterns. TLR-mediated activation of epithelial cells induces the production of defensins and cathelicidins — families of antimicrobial peptides.38
The skin produces the cathelicidin LL-37; the human β-defensins HBD-1, HBD-2, and HBD-3; and dermcidin. The inflammatory micromilieu initiated by interleukin-4, interleukin-13, and interleukin-10 down-regulates these antimicrobial peptides in the skin of patients with atopic dermatitis.40-43 For these reasons, it is difficult to manage microbial infections of the skin in patients with atopic dermatitis. Lesional and normal-looking skin is extensively colonized by bacteria such asStaphylococcus aureus or fungi such as malassezia. Patients with atopic dermatitis are predisposed to eczema herpeticum and eczema vaccinatum because of a reduced production of cathelicidin, which has potent antiviral activity.44,45
IMMUNOPATHOLOGIC MECHANISMS OF ATOPIC DERMATITIS
Initial Mechanisms of Skin Inflammation
Early-onset atopic dermatitis usually emerges in the absence of detectable IgE-mediated allergic sensitization,4 and in some children — mostly girls — such sensitization never occurs.5 The initial mechanisms that induce skin inflammation in patients with atopic dermatitis are unknown. They could entail neuropeptide-induced, irritation-induced, or pruritus-induced scratching, which releases proinflammatory cytokines from keratinocytes, or they could be T-cell–mediated but IgE-independent reactions to allergens present in the disturbed epidermal barrier or in food (so-called food-sensitive atopic dermatitis). Allergen-specific IgE is not a prerequisite, however, because the atopy patch test can show that aeroallergens applied under occluded skin induce a positive reaction in the absence of allergen-specific IgE.46,47
The Initiation Site of Sensitization
In patients with early-onset atopic dermatitis, IgE-mediated sensitization often occurs several weeks or months after the lesions appear,4 suggesting that the skin is the site of the sensitization. In animal models, repeated epidermal challenge with ovalbumin induces ovalbumin-specific IgE, respiratory allergy, and eczematous lesions at the application site.48 A similar process is likely in humans (Figure 2B).
Epidermal-barrier dysfunction is a prerequisite for the penetration of high-molecular-weight allergens in pollens, house-dust-mite products, microbes, and food. Molecules in pollens and some food allergens drive dendritic cells to enhance Th2 polarization.49,50 There are numerous T cells in skin (106 memory T cells per square centimeter of body-surface area), nearly twice the number in the circulation.51,52 Moreover, keratinocytes in atopic skin produce high levels of the interleukin-7–like thymic stromal lymphopoietin that signals dendritic cells to drive Th2 polarization.53 By inducing the production of large amounts of cytokines such as GM-CSF or chemokines, widespread skin inflammation can affect adaptive immunity,54 alter the phenotype of circulating monocytes,55-57and increase the production of prostaglandin E2 58 in atopic dermatitis. All these factors provide signals required for strong skin-driven Th2 polarization, and for this reason, the skin acts as the point of entry for atopic sensitization and may even deliver signals required for allergenic sensitization in the lung or the gut. The development of sensitization and atopic dermatitis in a bone marrow recipient after engraftment of hematopoietic stem cells from an atopic donor59provides support for the role of the hematopoietic system as a factor in addition to the genetically determined epidermal-barrier dysfunction in atopic dermatitis.
Antigen-specific IgE is the major recognition structure for allergens on mast cells and basophils. It may also be instrumental for the induction of allergen-specific tolerance or in antiinflammatory mechanisms,60 but whether such events underlie spontaneous remissions of atopic dermatitis remains to be explored.
Dendritic Cells
Epidermal dendritic cells in atopic dermatitis bear IgE61 and express its high-affinity receptor (FcεRI).62-64 In skin lesions, dendritic cells of the plasmacytoid lineage,65 which have potent antiviral activity by virtue of interferon-α production, are almost absent.66 In contrast, two populations of myeloid dendritic cells are present: Langerhans' cells and inflammatory dendritic epidermal cells.67 In atopic dermatitis, but not in other conditions, there is a high-density display of FcεRI by both types of cells. Langerhans' cells occur in normal skin, but inflammatory dendritic epidermal cells appear only in inflamed skin. They take up and present allergens to Th1 and Th2 cells, and possibly also to regulatory T cells.60 On ligation of FcεRI by IgE, Langerhans' cells produce interleukin-16, which recruits CD4+ T cells to the skin.68 Apart from interleukin-16, Langerhans' cells produce only a limited range of chemokines and almost no proinflammatory cytokines.69 On allergen capture, Langerhans' cells contribute to Th2 polarization by an unknown mechanism, and inflammatory dendritic epidermal cells lead to Th1 polarization by producing interleukin-12 and interleukin-18 and releasing proinflammatory cytokines. In the atopy patch test, high numbers of inflammatory dendritic epidermal cells invade the epidermis 72 hours after allergen challenge, and they and Langerhans' cells up-regulate their display of FcεRI.70
A Biphasic T-Cell–Mediated Disease
Allergen-specific CD4+ and CD8+ T cells can be isolated from the skin lesions of patients with atopic dermatitis. Inflammation in atopic dermatitis is biphasic: an initial Th2 phase precedes a chronic phase in which Th0 cells (cells that share some activities of both Th1 and Th2 cells) and Th1 cells are predominant (FIGURE 3
Acute and Chronic Phases of IgE and T-Cell–Mediated Atopic Dermatitis.).71 The Th2 cytokines interleukin-4, interleukin-5, and interleukin-13 predominate in the acute phase of the lesions, and in chronic lesions there is an increase of interferon-γ, interleukin-12, interleukin-5, and GM-CSF72; these changes are characteristic of Th1 and Th0 predominance. Th0 cells can differentiate into either Th1 or Th2 cells, depending on the predominant cytokine milieu. The increased expression of interferon-γ messenger RNA by Th1 cells follows a peak of interleukin-12 expression, which coincides with the appearance of inflammatory dendritic epidermal cells in the skin. Normal-looking skin in patients with atopic dermatitis harbors a mild infiltrate, strongly suggesting the presence of residual inflammation between flares.73
Recruitment of T cells into the skin is orchestrated by a complex network of mediators that contribute to chronic inflammation. Homeostatic and inflammatory chemokines produced by skin cells are involved in this process.74,75 Inflammatory cells and keratinocytes in the skin lesions express high levels of chemoattractants,76-78 and the keratinocyte-derived thymic stromal lymphopoietin induces dendritic cells to produce the Th2-cell–attracting thymus and activation-regulated chemokine, TARC/CCL17. In this way, they may amplify and sustain the allergic response and the generation of interferon-γ–producing cytotoxic T cells,79 as suggested by in vitro studies. Interferon-γ produced by Th1 cells has been implicated in the apoptosis of keratinocytes induced by the cell-death receptor Fas.80
The role of regulatory T cells in atopic dermatitis81 has also been examined. High levels of expression of the alpha chain of the interleukin-2 receptor (CD25) and the transcription factor FOXP3 are characteristic of these cells. There is an increased pool of circulating regulatory T cells in atopic dermatitis,82 but the skin lesions are devoid of functional regulatory T cells.83 The complexity of the regulatory T-cell compartment is not yet fully understood, and the role of regulatory T cells in the regulation of chronic inflammatory skin disease is elusive.
Staphylococcus aureus
Suppression of the innate immune system of the skin by the inflammatory micromilieu of atopic dermatitis explains the colonization of the skin by S. aureus in more than 90% of patients with atopic dermatitis. 83 This feature contributes to allergic sensitization and inflammation (FIGURE 4
Multiple Pathways ofStaphylococcus aureus–Driven Sensitization and Inflammation.). Scratching increases the binding of S. aureus to the skin, and the increased amount of S. aureus–derived ceramidase can aggravate the defect in the skin barrier. S. aureus enterotoxins84 increase the inflammation in atopic dermatitis and provoke the generation of enterotoxin-specific IgE, which correlates with the severity of the disease. 85 These enterotoxins interact directly with class II molecules of the major histocompatibility complex and the beta chain of the T-cell receptor to induce an antigen-independent proliferation of T cells. They also up-regulate the expression of the skin-homing receptor cutaneous lymphocyte-associated antigen on T cells and the production of keratinocyte-derived chemokines that recruit T cells. By inducing the competing β-isoform of the glucocorticoid receptor in mononuclear cells, enterotoxins contribute to the emergence of a resistance to local corticosteroid treatment. S. aureus enterotoxins also induce the expression of the glucocorticoid-induced protein ligand related to the tumor necrosis factor receptor on antigen-presenting cells, resulting in an inhibition of the suppressive activity of regulatory T cells.86
Mechanism of Pruritus
The most important symptom in atopic dermatitis is persistent pruritus, which impairs the patient's quality of life. The lack of effect of antihistamines argues against a role of histamine in causing atopic dermatitis–related pruritus.87 Neuropeptides, proteases, kinins, and cytokines induce itching. Interleukin-31 is a cytokine produced by T cells that increases the survival of hematopoietic cells and stimulates the production of inflammatory cytokines by epithelial cells. It is strongly pruritogenic, and both interleukin-31 and its receptor are overexpressed in lesional skin.88-90Moreover, interleukin-31 is up-regulated by exposure to staphylococcal exotoxins in vitro. These findings implicate interleukin-31 as a major factor in the genesis of pruritus in atopic dermatitis.
AUTOIMMUNITY IN ATOPIC DERMATITIS
In addition to IgE antibodies against food and aeroallergens, serum specimens from patients with severe atopic dermatitis contain IgE antibodies against proteins from keratinocytes and endothelial cells such as manganese superoxide dismutase and calcium-binding proteins.91,92 The serum levels of these IgE autoantibodies correlate with disease severity. Scratching probably releases intracellular proteins from keratinocytes. These proteins could be molecular mimics of microbial structures and thus could induce IgE autoantibodies.93 About 25% of adults with atopic dermatitis have IgE antibodies against self-proteins.94 In these patients, early-onset atopic dermatitis, intense pruritus, recurrent bacterial skin infections, and high serum IgE levels are hallmarks of the disease. Furthermore, IgE antibodies against self-proteins can be detected in patients with atopic dermatitis as early as 1 year of age.94 Some autoallergens in skin are also strong inducers of Th1 responses.92 IgE antibodies in atopic dermatitis can be induced by environmental allergens, but IgE antibodies against autoantigens in the skin can perpetuate the allergic inflammation. Thus, atopic dermatitis seems to stand at the frontier between allergy and autoimmunity.
A UNIFYING HYPOTHESIS
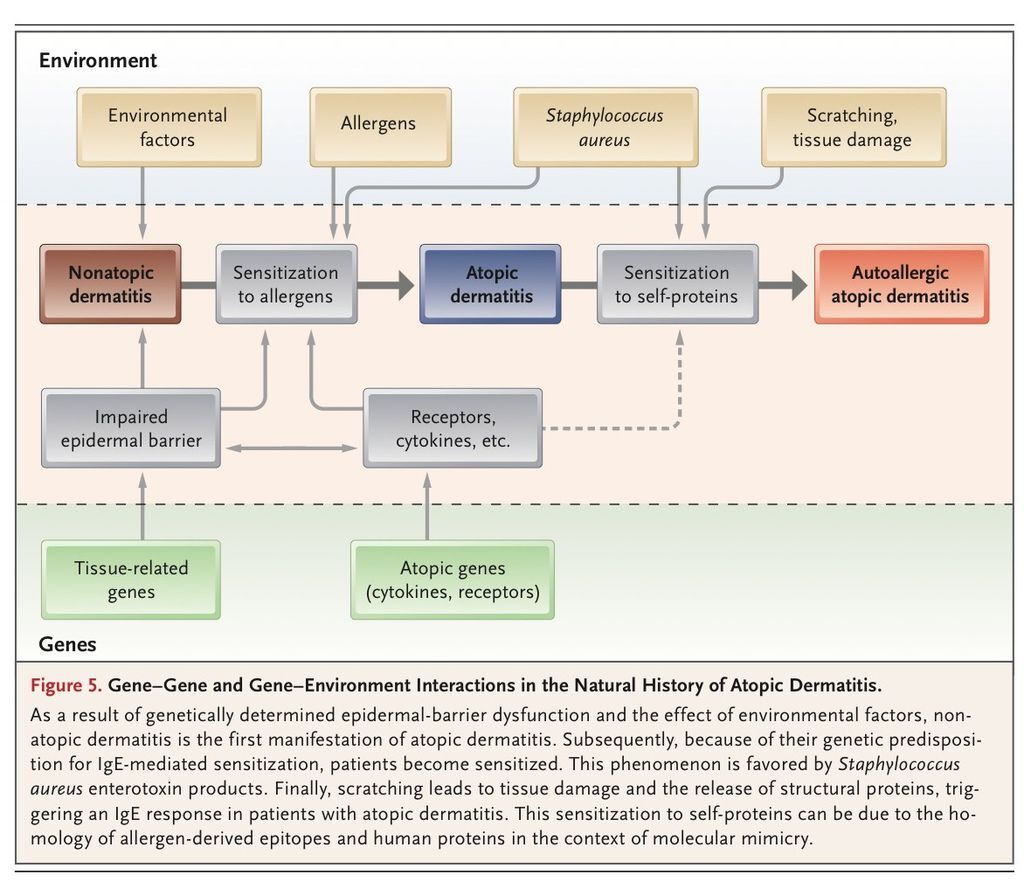
One classification distinguishes an IgE-associated form of atopic dermatitis (i.e., true atopic dermatitis, formerly called extrinsic atopic dermatitis) from a non–IgE-associated form (“nonatopic” dermatitis, formerly called intrinsic atopic dermatitis).95 This division implies that nonatopic dermatitis and atopic dermatitis are two different diseases. However, since dry skin is an important sign of both conditions, and the absence of IgE-mediated sensitization may be only a transient factor, there is a need to reconcile these divergent hypotheses. A new picture emerges from recent findings, in which the natural history of atopic dermatitis has three phases (FIGURE 5
Gene–Gene and Gene–Environment Interactions in the Natural History of Atopic Dermatitis.). The initial phase is the nonatopic form of dermatitis in early infancy, when sensitization has not yet occurred. Next, in 60 to 80% of patients, genetic factors influence the induction of IgE-mediated sensitization to food, environmental allergens, or both — this is the transition to true atopic dermatitis. Third, scratching damages skin cells, which release autoantigens that induce IgE autoantibodies in a substantial proportion of patients with atopic dermatitis.
CLINICAL IMPLICATIONS
Since the barrier dysfunction of the skin and chronic inflammation are characteristic of atopic dermatitis, long-term clinical management should emphasize prevention, intensified and individually adapted skin care, reduction of bacterial colonization by means of local application of lotions containing antiseptics such as triclosan and chlorhexidine, and — most important — the control of inflammation by the regular use of topical corticosteroids or topical calcineurin inhibitors. In children, before and after the diagnosis of IgE-mediated sensitization, measures that prevent exposure to allergens should be beneficial. The current therapy of atopic dermatitis is reactive — treating the flares — but management should include early and proactive intervention with effective and continuous control of the skin inflammation and S. aureuscolonization. This strategy has proved to be effective in reducing the number of flares.96 When applied early in infancy, it could potentially help to reduce later sensitization to environmental antigens and autoallergens.
CONCLUSIONS
Recent insights into the genetic and immunologic mechanisms that drive cutaneous inflammation in atopic dermatitis have led to a better understanding of the natural history of this disease and have highlighted the critical role of the epidermal-barrier function and the immune system. Both contribute to IgE-mediated sensitization and should be considered as major targets for therapy. New developments aimed specifically at the molecular defects in the stratum corneum could provide a customized way to improve the barrier function. Early and proactive management could improve the outcome and quality of life for patients with atopic dermatitis.







 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 