Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults. Report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society.
[EXCERPTS摘要]
Major Recommendations
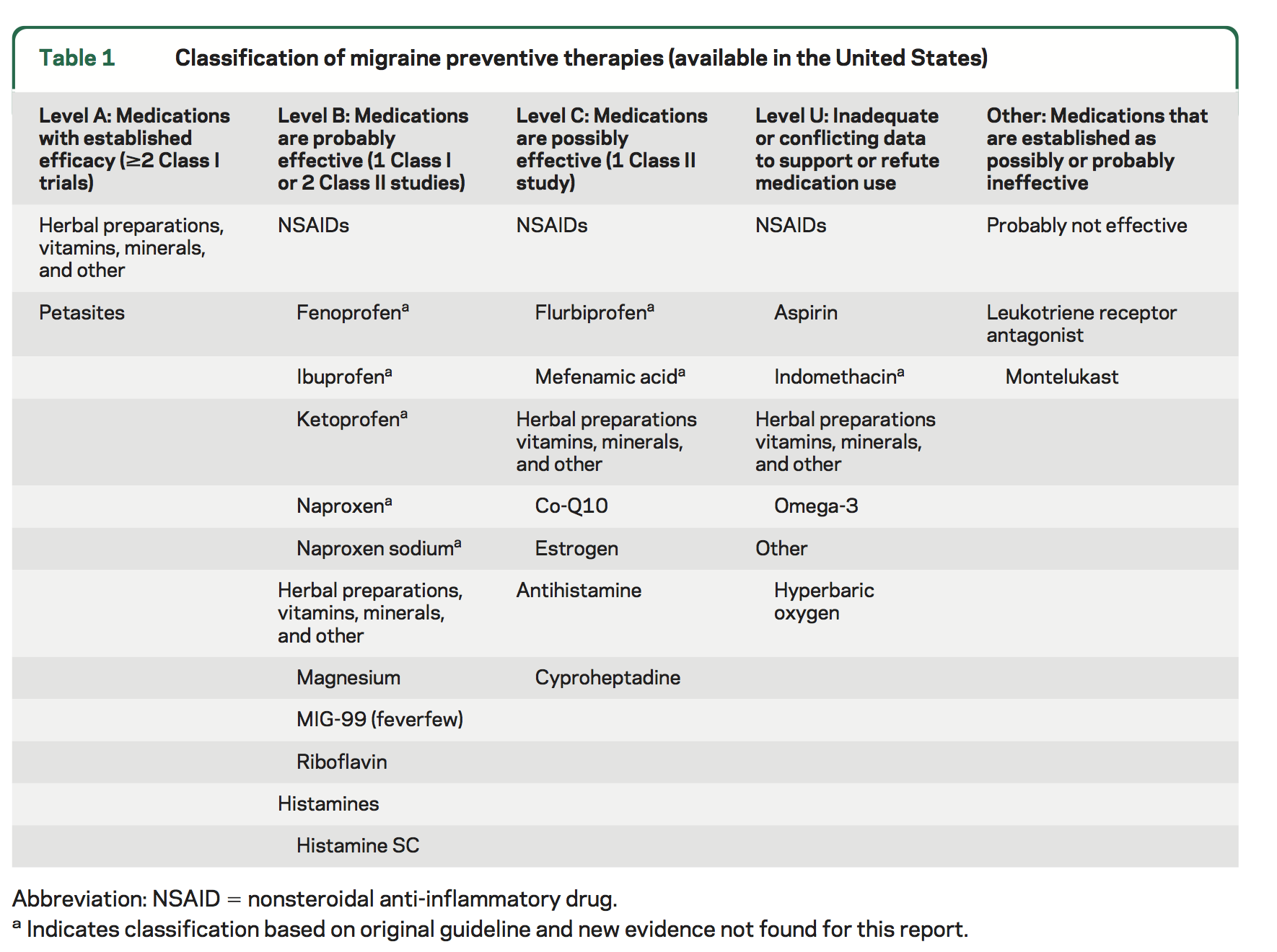
Level A. The following therapy is established as effective and should be offered for migraine prevention:
Petasites (butterbur)
Level B. The following therapies are probably effective and should be considered for migraine prevention:
NSAIDs: fenoprofen, ibuprofen, ketoprofen, naproxen, naproxen sodium
Herbal therapies, vitamins, and minerals: riboflavin, magnesium, MIG-99 (feverfew)
Histamines: histamine subcutaneous (SC)
Level C. The following therapies are possibly effective and may be considered for migraine prevention:
NSAIDs: flurbiprofen, mefenamic acid
Herbal therapies, vitamins, and minerals: Co-Q10, estrogen
Antihistamines: cyproheptadine
Level U. Evidence is inadequate or conflicting to support or refute the use of the following therapies for migraine prevention:
NSAIDs: aspirin, indomethacin
Herbal therapies, vitamins, and minerals: omega-3
Other: HBO
Level B negative. The following therapy is probably ineffective and should not be considered for migraine prevention:
Leukotriene receptor antagonists: montelukast
Clinical Context
In a previous epidemiologic study, 38.7% of study participants had ever used a migraine preventive treatment, of which only 12.4% were current users and 17.2% were coincident users (taking a migraine preventive treatment for other reasons). The proportion of those who use nonsteroidal anti-inflammatory drugs (NSAIDs) or individual complementary treatments specifically for migraine prevention is unclear at this time, and is a topic which warrants further study. Additionally, the treatments reviewed herein are those available in the United States. In other countries, treatments may not be available commercially or may be available in other dosages or in other preparations or combinations. Therefore, the results from this and other guidelines are limited to those treatments available in the United States.
Additionally, studies assessing the efficacy of NSAIDs and complementary treatments for migraine prevention are limited and should be considered relative to other available pharmacologic therapies reviewed in a separate guideline. Silberstein and colleagues report divalproex sodium, sodium valproate, topiramate, metoprolol, propranolol, and timolol are effective for migraine prevention and should be offered to patients with migraine to reduce migraine attack frequency and severity (Level A).
Additionally, the clinical evidence for NSAIDs and complementary treatments for migraine prevention should be reviewed with caution because there are clear discrepancies in how patients were selected for study inclusion; how severe, frequent, or disabling their attacks were; and how severity was assessed. Also, these treatments are unregulated. There are few or no studies on how these medications should be taken—specifically relative to dosing strategies and coadministration with other prescription pharmacologic treatments. When patients are instructed or choose to take NSAIDs or complementary treatments for migraine prevention, it is important that they be followed over the course of treatment so dosing and titration modifications and adverse effect (AE) risk can be monitored. Prospective long-term safety of many of these agents is not well studied specifically regarding their use as preventive migraine treatments.
It is reasonable also for clinicians to inquire about the doses being used and frequency of use of NSAIDs and complementary treatments. Frequent medication use or high dose levels may increase the risk of headache progression or medication overuse, which may lead to other secondary health complications (e.g., gastrointestinal upset/bleeding with aspirin or NSAIDs or headache rebound with discontinuation of feverfew). Complete review and disclosure of coexisting conditions are warranted, as complementary or pharmacologic therapies taken for coexisting conditions (e.g., depression) may exacerbate headache. Because migraine is frequent in women of childbearing age, the potential for adverse fetal effects related to migraine prevention strategies is of particular concern. Little has been done to establish the long-term safety and efficacy of these agents during pregnancy or breastfeeding.
Additionally, when patients have unlimited access to over-the-counter medications, they may be unaware of the continued need for routine physician follow-up for a chronic illness such as migraine, as illness severity may progress or improve, often warranting medication changes (see table e-1 of the data supplement [see the "Availability of Companion Documents" field]). It also is important for patients to understand the magnitude of benefit that can be expected from preventive migraine therapies; moreover, patient education about migraine and appropriate management is important in successful patient care. For some patients, a 35% reduction in headache frequency or intensity may be deemed an insufficient level of improvement, thus leading them to risk dose escalation. Additionally, patients with migraine may need to be educated about appropriate use and risks of these agents.
Finally, recent studies suggest that some medications used for migraine may offer long-term protection against headache progression whereas other agents may elevate progression risk. Specifically, one epidemiologic study assessing medication use in the general migraine population reports that aspirin or ibuprofen use may protect against progression from episodic to chronic headache conditions. In contrast, opioid use was positively associated with chronic headache conditions. Although conclusions are preliminary regarding the benefits and risks of selected agents for long-term use, studies suggest that these agents may play a significant role in headache progression and patterns, lending further emphasis to the importance of following patients closely, including regular assessment of NSAIDs, and other complementary treatments for migraine prevention.
Classification of Recommendations [and Evidence] [available online]
Patient Resources
NSAIDS and complementary treatments for migraine prevention in adults. AAN summary of evidence-based guideline for patients and their families. St. Paul (MN): American Academy of Neurology. 2012. 2 p. Electronic copies: Available in English and Spanish in Portable Document Format (PDF) from the American Academy of Neurology (AAN) Web site.
[Link to full-text guideline: Neurology PDF | National Guideline Clearinghouse version online]





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 