This past year marked the 20th anniversary of the discovery of the Janus kinase (JAK)–signal transducer and activator of transcription (STAT) pathway.1 Arising from efforts to understand the molecular mechanisms of interferon action, the elucidation of this pathway has provided many insights into disease mechanisms and has become the basis for new pharmacologic agents. It is therefore an appropriate time to take stock of our knowledge of this pathway and to consider the ways in which these insights are affecting the practice of medicine.
CYTOKINES AND JAK–STAT SIGNALING
Interferons, erythropoietin, growth hormone, and prolactin, all discovered more than half a century ago, are a few of the dozens of cytokines that have been found to play critical roles in cell growth and differentiation, metabolism, hematopoiesis, host defense, and immunoregulation. It would be hard to overstate the influence of cytokines — as well as anticytokine and anticytokine-receptor antibodies — on medicine.
Understanding the molecular basis of cytokine action has been of great interest, not only for the purpose of basic research but also to further our understanding of disease pathogenesis and the development of new therapies. The term “cytokine” can be confusing because it refers to a broad collection of secreted factors that belong to different structural families and that use different types of receptors and distinct modes of signaling. One large subgroup, the type I and type II cytokine-receptor superfamily, encompasses receptors that bind interferons, many (but not all) interleukins, and colony-stimulating factors. These cytokines all use the same mechanism of signal transduction: the JAK–STAT pathway.2Because erythropoietin, thrombopoietin, growth hormone, prolactin, and leptin use the same class of receptor, they can also be included in this family of cytokines. Other cytokines, such as tumor necrosis factor, interleukins 1 and 8, transforming growth factor β, and macrophage colony-stimulating factor, bind different classes of receptors and do not use the JAK–STAT pathway as their essential mode of signaling.3
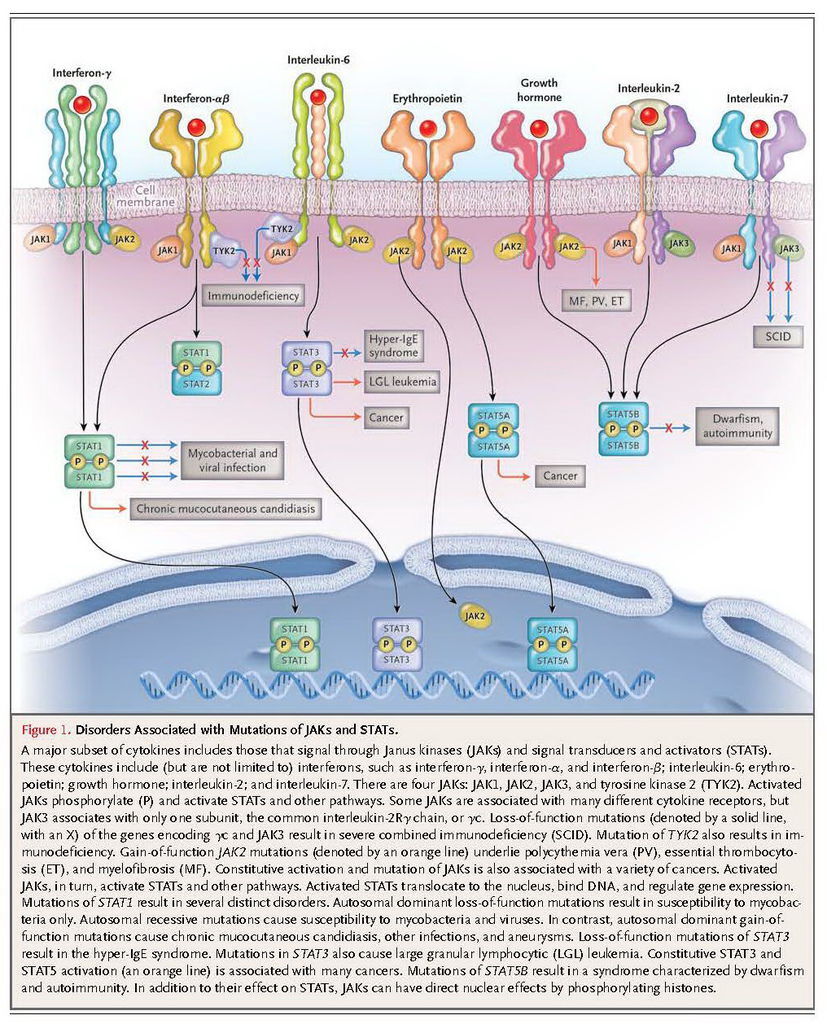
Four JAKs — JAK1, JAK2, JAK3, and tyrosine kinase 2 (TYK2) — selectively associate with the cytoplasmic domains of various cytokine receptors (Figure 1). JAK3 and TYK2 are primarily important for immune responses. JAK1 and JAK2 have broad functions, with roles that range from host defense and hematopoiesis to growth and neural development; deletion of Jak1 or Jak2 is lethal in mice.2 Cytokine binding activates JAKs, which in turn phosphorylate cytokine receptors. This process allows the selective binding of members of the STAT family: STAT1, STAT2, STAT3, STAT4, STAT5A, STAT5B, and STAT6. These DNA-binding proteins become tyrosine-phosphorylated, which allows them to dimerize, translocate to the nucleus, and regulate gene expression. In mice, complete deficiency of Stat3 or Stat5a and Stat5b is lethal, a finding that is consistent with the broad, critical functions of these proteins. STAT1, STAT2, STAT4, and STAT6 have more restricted functions, playing roles in host defense and immunoregulation.
PRIMARY IMMUNODEFICIENCY
Dramatic examples of the importance of JAKs and STATs have come from studying patients with primary immunodeficiencies.4 Among the four JAKs, mutations of JAK3 and TYK2 are known causes of primary immunodeficiency, including severe combined immune deficiency (SCID). Mutations of STAT1, STAT3, and STAT5B are also associated with specific clinical syndromes.
SCID and JAK3 Mutations
The cause of SCID is impaired development or function of lymphocytes. The receptor chain shared by interleukins 2, 4, 7, 9, 15, and 21 is the common interleukin-2Rγ chain, γc; the gene encoding this protein is located at Xq13. Mutations in the gene encoding interleukin-2Rγ (IL2RG) cause X-linked SCID, which is characterized by markedly diminished T-cell and natural killer (NK) cell numbers and function but preserved B-cell numbers with impaired function (T−B+NK−). The dependence of γc signaling on JAK3 was established when it was determined that most patients who have T−B+NK− SCID without γc mutations have JAK3 mutations.4 In contrast, mutations in the interleukin-7 receptor α chain specifically impair T-cell development, leading to T−B+NK+ SCID, since interleukin-15 signaling, which is required for NK-cell development, is maintained.
TYK2
There are only two instances in which autosomal recessive mutations in TYK2 have been reported in humans, and their phenotypes differed. One child had atopic dermatitis and moderately elevated IgE levels as well as severe bacterial, viral, and fungal infections.5 The other child had severe infection after vaccination with bacille Calmette–Guérin (BCG), neurobrucellosis, and infection with herpes simplex virus (HSV), but only very minor elevations in IgE levels and no atopy.6
STAT1
Human mutations in STAT1 were first identified in patients with disseminated BCG infection or nontuberculous mycobacterial infection in childhood.7 These mutations were dominant-negative for interferon-γ signaling but recessive for interferon-α signaling, which resulted in susceptibility to mycobacterial infection but normal viral control. Whereas dominant-negative mutations lead to relatively mild mycobacterial disease, complete STAT1 deficiency blocks both interferon-γ and interferon-α signaling. Patients with complete deficiency are susceptible to viral and mycobacterial infections, which usually lead to early death.8 Between the complete recessive loss of interferon-γ and interferon-α signaling and the presence of dominant-negative inhibition of interferon-γ signaling alone are the recessive hypomorphic mutations.9 These biallelic mutations result in recurrent infections with intramacrophagic bacteria (e.g., salmonella, BCG, and nontuberculous mycobacteria) and herpes viruses (e.g., HSV, cytomegalovirus, and varicella–zoster virus), but with treatment there is adequate immunity to support survival.10
Heterozygous gain-of-function or hypermorphic mutations that cause chronic mucocutaneous candidiasis have recently been described.11,12 Excessive STAT1 activation causes exaggerated responses to interferon-γ and inhibits the production of interleukin-17. Patients with hypermorphic mutations in STAT1are susceptible to other complications, including autoimmunity, cerebral aneurysms, and squamous-cell carcinoma. These mutations are also associated with the disseminated dimorphic yeast infections coccidioidomycosis and histoplasmosis and with the IPEX-like (immune dysregulation, polyendocrinopathy, enteropathy, X-linked) syndrome, in which the transcription factor FOXP3 and regulatory T cells are intact.13 It will be of interest to learn precisely how hyperactivating mutations inSTAT1 are related to epithelial, invasive, and opportunistic infections as well as cancer and vascular disease.
STAT3
The search for the genetic cause of the autosomal dominant hyper-IgE (or Job's) syndrome culminated in the surprising identification of dominant-negative mutations in STAT3.14,15 The hyper-IgE syndrome is characterized by eczema, staphylococcal boils, cyst-forming pneumonias, chronic mucocutaneous candidiasis, and extremely high levels of IgE, along with numerous nonimmunologic features, including scoliosis, fractures, characteristic facies, failure of primary tooth deciduation, and coronary-artery aneurysms.16,17 The mutations that cause this complex, multisystem disease are located mainly in the DNA-binding and Src homology 2 (SH2) domains of STAT3, leading to interference with the functioning of the normal allele. STAT3 was known to be crucial for cytokines that drive the differentiation of interleukin-17–producing T cells. Accordingly, interleukin-17–producing cells are diminished in patients with the hyper-IgE syndrome, providing the first evidence of the critical role this cytokine plays in humans.18-20 Because STAT3 is also critical for CD8 T-cell memory, STAT3 mutations are associated with recurrent infection with varicella–zoster virus and increased circulating levels of Epstein–Barr virus.21However, STAT3 mediates signaling through at least six classes of receptors,4 making it a multifaceted disease with features beyond immune-cell defects, including coronary-artery aneurysms without coronary atherosclerosis.
STAT5
Recessive STAT5B deficiency results in immunodeficiency, autoimmunity, and growth failure.22 Patients with STAT5B deficiency have severe opportunistic infections, variable lymphocyte counts, and normal-to-high levels of immunoglobulins. These effects are explained by the roles STAT5B plays in interleukin-2 and growth-hormone signaling.23,24 STAT5B is important for the expression of FOXP3 and interleukin-2Rα (CD25) and for the differentiation of regulatory T cells.25 STAT5 also down-regulates interleukin-17,26 and both factors underlie the autoimmunity associated with STAT5B deficiency.
IMMUNOREGULATION AND GENETIC LINKS TO IMMUNE-MEDIATED DISEASE
Animal models have provided powerful evidence that the JAK–STAT pathway and the cytokines that use this pathway play critical roles in the pathogenesis of autoimmunity, allergy, asthma, and other immune-mediated diseases. More recently, genomewide association studies have buttressed the argument that cytokines and cytokine signaling are relevant to human disease. Inherited variation in genes encoding cytokines and cytokine receptors and their respective JAKs and STATs are associated with a significantly increased risk of immune-mediated disease.
Multiple genes in the interleukin-23 signaling pathway contribute to a surprising range of autoimmune diseases, including inflammatory bowel disease, psoriasis, ankylosing spondylitis, and Behçet's disease.27-29 Polymorphisms of JAK2 and STAT3 are associated with these disorders. Similarly, STAT4 is activated by interleukin-12 and interferon type I, and polymorphisms of STAT4 are associated with systemic lupus erythematosus, rheumatoid arthritis, and Sjögren's syndrome.30 Interleukins 4 and 13 activate STAT6, and STAT6 polymorphisms are associated with elevated IgE levels and atopic dermatitis.31,32
CANCER
In addition to having roles in host defense and autoimmunity, the JAKs have critical roles in hematologic cancers.33 The initial evidence of these roles derived from the recognition of the constitutive activation of JAKs and STATs in patients with cancer.34,35 JAK2 mutations are associated with myeloproliferative neoplasms, clonal cancers arising from hematopoietic progenitor cells, which include polycythemia vera, essential thrombocythemia, and primary myelofibrosis.36-39 The most frequent JAK2 mutation, V617F, occurs in more than 95% of patients with polycythemia vera and in 32 to 57% of patients with essential thrombocythemia or primary myelofibrosis. The selective association of the V617F mutation with myeloproliferative neoplasms is diagnostically useful, helping to distinguish these disorders from nonmalignant syndromes that affect blood counts. Less frequently, myeloproliferative neoplasms harbor mutations in exon 12 of JAK2 in the absence of the V617F mutation; in these instances, patients typically have an isolated erythrocytosis, which may represent a distinct clinical syndrome.40
The presence of a myeloproliferative neoplasm in a first-degree relative increases the risk of disease by a factor of 5 to 7.41 An extended germline haplotype encompassing the JAK2 locus is responsible for much of this familial predisposition, increasing the risk of disease by a factor of 3 to 4.42-44 When a myeloproliferative neoplasm develops, the somatic V617F mutation is present preferentially on the germline-encoded JAK2 allele that confers susceptibility, suggesting that this allele harbors cis-acting elements that increase the likelihood of the V617F mutation.
The JAK2 V617F mutant is located within the JH2 “kinaselike” domain,45,46 which is catalytically active and can phosphorylate and activate the kinase domain.47 Thus, the mutation creates a constitutively active kinase that can render hematopoietic cells independent of exogenous growth factors, thereby causing polycythemia vera and other myeloproliferative processes in mice.48,49 These observations establish the oncogenic importance of the V617F mutation.
Somatically acquired mutations in JAK2 have been detected in high-risk patients with B-cell acute lymphoblastic leukemia (ALL) (9%) and in patients with B-cell ALL associated with Down's syndrome (34%), most often affecting the R683 residue.50-52 Other JAKs can be activated by mutations in hematologic cancers, including JAK1 in patients with T-cell ALL, patients with B-cell ALL who have a poor prognosis, and patients with acute myeloid leukemia,52-55 and JAK3 in patients with T-cell ALL, patients with adult T-cell leukemia or lymphoma, and patients with NK-cell or T-cell lymphoma.56-58
The function of receptors that associate with JAKs can also be altered by chromosomal rearrangements or mutations in cancer, leading to constitutive JAK activity. Activating mutations affecting the thrombopoietin receptor MPL occur in approximately 9% of patients with myelofibrosis, all of whom lack the JAK2 V617F mutation, which leads to the constitutive activation of JAK2 by the MPL receptor.59 In approximately 50% of patients with B-cell ALL, the cytokine receptor CRLF2, which binds JAK2, is over-expressed by chromosomal rearrangements, including 34% of patients with Down's syndrome and 9% of high-risk children with B-cell ALL.52,60-62 Many leukemias with alterations in CRLF2 also have JAK2mutations, suggesting that this receptor functions as a platform for constitutive signaling by mutant JAK2. Approximately 10% of patients with T-cell ALL have mutant interleukin-7 receptor α subunits, leading to constitutive JAK1 activation.63,64 Gain-of-function mutations of the granulocyte colony-stimulating factor receptor are associated with acute myeloid leukemia in conjunction with severe congenital neutropenia.65 Outside the hematopoietic lineage, in-frame deletions affecting glycoprotein 130, the signaling component of the interleukin-6 receptor, are present in 60% of patients with inflammatory hepatocellular adenomas, causing JAK2 activation.66
JAKs can also be activated by autocrine cytokine secretion in several subtypes of lymphoma. In primary mediastinal B-cell lymphoma and Hodgkin's lymphoma, autocrine interleukin-13 signaling activates JAK2, and in 30 to 50% of patients, its activity is further intensified by amplification of the JAK2locus.67,68 Thus, JAK2 inhibition is lethal to the cell lines for both types of lymphoma.68
Autocrine secretion of interleukins 6 and 10 activates JAKs in the activated B-cell-like (ABC) subtype of diffuse large-B-cell lymphoma, promoting the survival of malignant cells.69-71 In many cases, this autocrine cytokine loop is initiated by activating mutations affecting MYD88, an adaptor protein in toll-like receptor signaling.70 The most common MYD88 mutant, termed L265P, occurs in 29% of patients with ABC diffuse large-B-cell lymphoma, and it is also present in 36% of patients with primary central nervous system lymphoma72 and 69% of patients with leg-type primary cutaneous lymphoma, both of which phenotypically resemble ABC diffuse large-B-cell lymphoma. MYD88 L265P is also common in Waldenström's macroglobulinemia73 (90% of cases) and occurs in a subset of marginal-zone lymphomas70,74 (10 to 11% of cases) and chronic lymphocytic leukemias75,76 (3 to 10% of cases). In ABC diffuse large-B-cell lymphoma, this MYD88 mutant spontaneously coordinates an active signaling complex composed of the interleukin-1–associated kinases IRAK1 and IRAK4, thereby engaging the nuclear factor κB and p38 MAP kinase pathways and leading to the synthesis of interleukin-6 and interleukin-10 and autocrine JAK activation.70
The downstream effectors of JAK signaling in hematopoietic cancer include the PI-3 kinase and Ras pathways and the STAT transcription factors.33 The JAK2 V617F mutation does not cause myeloproliferative disease in mice lacking STAT5,77-79 and STAT3 is required for the survival of ABC diffuse large-B-cell lymphoma cells.69,80 STAT3 is mutated in 40% of large granular leukemias,81 with its SH2 domain altered at some of the same positions in which it is altered in patients with the hyper-IgE syndrome, albeit with different amino acid substitutions. Constitutive STAT activation, which is common in epithelial, liver, and breast cancers, fosters the proliferation and survival of malignant cells and tumor-promoting inflammation while mitigating antitumor immunity.82-85 The complex role of STATs makes them a challenging but important subject for research.
In the past few years, researchers have uncovered a noncanonical, epigenetic role for JAK signaling in the nucleus in leukemias and lymphomas.68,86 JAK2 translocates into the nucleus in leukemias with the V617F mutation and in primary mediastinal B-cell lymphoma and Hodgkin's lymphoma with amplification of the JAK2 locus. There, JAK2 phosphorylates the histone H3 tail on tyrosine 41, counteracting the formation of heterochromatin and promoting gene expression, including expression of the oncogeneMYC in both types of lymphoma.68,86 The JAK2 amplicon in these lymphomas also includes JMJD2C,which encodes a chromatin modifier that counteracts the formation of heterochromatin and cooperates with JAK2 in activating genes epigenetically.68
PHARMACOLOGIC INHIBITION OF CYTOKINE SIGNALING WITH JAK INHIBITORS
Hematologic Disease
The discovery of JAK2 mutations in myeloproliferative neoplasms prompted the evaluation of JAK inhibitors for the treatment of patients with these neoplasms. The JAK1–JAK2 inhibitor ruxolitinib was initially tested in clinical trials involving 153 patients with myelofibrosis.87-89 Evidence that the drug blocked JAK signaling included loss of STAT3 phosphorylation and expression of interleukin-6 and tumor necrosis factor (TNF). These alterations occurred in patients with and those without the V617F mutation, indicating either that wild-type JAK2 plays a pathogenetic role in myelofibrosis or that the blockade of other kinases contributes to the therapeutic effect. Overall, among patients with splenomegaly, spleen size decreased by more than half in 44%, and the decrease in size was accompanied by a marked reduction of associated symptoms.88,89 Ruxolitinib has now been approved by the Food and Drug Administration for the treatment of myeloproliferative neoplasms (the side effects of ruxolitinib and other JAK inhibitors are discussed, below).
The genetic and functional evidence described above with regard to lymphomas provides an impetus for the evaluation of JAK2 inhibitors in primary mediastinal B-cell lymphoma and Hodgkin's lymphoma and for the evaluation of JAK1 inhibitors in ABC diffuse large-B-cell lymphoma.68 In ABC diffuse large-B-cell lymphoma cells, the MYD88 L265P mutant not only induces autocrine secretion of interleukins 6 and 10, which enhance survival, but also induces autocrine secretion of interferon-β.70,90 Interferon-β can limit tumor growth, but its production is held in check by the transcription factor interferon regulatory factor 4 (IRF4). Lenalidomide, a drug that has shown activity against ABC diffuse large-B-cell lymphoma in early-phase clinical trials,91 interferes with IRF4 expression, thereby unleashing interferon-β secretion and killing the lymphoma cells. IRF4 expression can also be suppressed by the BTK kinase inhibitor ibrutinib, which blocks the chronic active B-cell-receptor signaling that typifies this lymphoma subtype. The synergistic activity of ibrutinib and lenalidomide in killing ABC diffuse large-B-cell lymphoma cells, both in vitro and in xenografts, sets the stage for clinical trials of this drug combination.
Autoimmunity
Because of the importance of cytokines in autoimmune diseases, the targeting of JAKs appeared to be a logical strategy to pursue in treating these disorders as well. In fact, a number of JAK inhibitors are in various stages of preclinical development or are being tested in clinical trials (Table 1.). One JAK inhibitor, tofacitinib, has been approved for the treatment of arthritis in patients for whom methotrexate is not effective.
Tofacitinib inhibits JAK3 and JAK1 and, to a lesser extent, JAK2 but has little effect on other kinases.92 Functionally, tofacitinib affects both innate and adaptive immune responses and was found to be effective in preclinical models.93 It has also shown efficacy in the treatment of rheumatoid arthritis in phase 3 trials.94,95 In one study, patients with rheumatoid arthritis who had been receiving methotrexate were given tofacitinib, adalimumab (a TNF-α inhibitor), or placebo. The response rates for patients receiving tofacitinib were similar to the rates among patients receiving adalimumab. In a second study, patients with rheumatoid arthritis in whom conventional therapies had not been effective were found to have a response to tofacitinib. The efficacy of tofacitinib in ulcerative colitis and psoriasis has also been reported recently.96,97
Patients receiving tofacitinib had an increased incidence of infection, including tuberculosis and herpes zoster. Anemia and neutropenia were also noted, presumably in relation to JAK2 inhibition and interference with signaling by erythropoietin and other colony-stimulating factors. Increases in lipid levels were also seen, a response that may be related to the blockade of interleukin-6 signaling. JAKs are important for NK-cell–mediated resistance to tumors, but it remains to be determined whether JAK inhibitors augment the risk of cancer in rheumatoid arthritis.98 The side effects of other first-generation JAK inhibitors (ruxolitinib and baricitinib) appear to be similar, and the described side effects are largely related to the mechanism of action — that is, inhibition of cytokine action. As small molecules, these drugs may also have unrelated, off-target effects.
Cytokines are important in a wide range of diseases, from cancer to immune-mediated disease, and the potential usefulness of JAK inhibitors is equally broad. To what extent JAK inhibitors will be used in conjunction with or in place of other immunomodulatory agents remains to be seen. The inhibitors now in clinical use do not selectively inhibit a single JAK; the question of whether more selective, second-generation agents will be as efficacious but with reduced adverse effects is an important area for future investigation. The comparative safety profiles of first- and second-generation JAK inhibitors will become better understood as they are tested in larger studies in various clinical settings. (Table 1 reviews the cytokines and mutations associated with activation of JAKs, as well as the drugs that inhibit activation.)
On the basis of their critical functions, it would seem logical to target STATs in a variety of disorders, from autoimmune diseases and allergies to cancer and atherosclerosis.99,100 Despite considerable effort, clinically useful STAT inhibitors have yet to become a reality. Unlike JAKs, which are enzymes, STATs have been more challenging targets; because of their enormous potential, work in the area of STAT inhibitors continues. (Table 2 reviews the cytokines and the mutations that influence the activation of STATs.)
CONCLUSIONS
What began as an effort to understand basic mechanisms in interferon-mediated gene regulation has resulted in a new framework for the study of cell signaling. Moreover, the discovery of this pathway has clarified the pathophysiology of diseases ranging from primary immunodeficiencies to various cancers. The prospect of targeting the JAK–STAT pathway is now a reality, and we are probably just beginning to see the spectrum of diseases for which such targeting might be useful.





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 