N Engl J Med 2014; 370:444-454January 30, 2014DOI: 10.1056/NEJMra1208705
Patients in intensive care units (ICUs) are treated with many interventions (most notably endotracheal intubation and invasive mechanical ventilation) that are observed or perceived to be distressing. Pain is the most common memory patients have of their ICU stay.1 Agitation can precipitate accidental removal of endotracheal tubes or of intravascular catheters used for monitoring or administration of life-sustaining medications. Consequently, sedatives and analgesics are among the most commonly administered drugs in ICUs.
Early intensive care practice evolved from intraoperative anesthetic care at a time when mechanical ventilation was delivered by rudimentary machines that were not capable of synchronizing with patients' respiratory efforts. As a result, deep sedation was commonly used until a patient was able to breathe without assistance. Developments over the past 30 years, including microprocessor-controlled ventilators that synchronize with patients' own respiratory efforts and new, shorter-acting sedative and analgesic medications, have dramatically changed this approach. Equally important has been the recognition that pain, oversedation, and delirium are issues that if undetected and untreated are distressing to patients and associated with increased morbidity and mortality.
Just as the concept of the “triad of anesthesia” underscores the pharmacodynamic interactions among hypnotics, analgesics, and muscle relaxants and the recognition that the simultaneous administration of agents of each class permits the use of lower doses of drugs of all classes, the concept of the “ICU triad” recognizes that pain, agitation, and delirium — and therefore approaches to their management — are inextricably linked (Figure 1
FIGURE 1
Causes and Interactions of Pain, Agitation, and Delirium.
). According to the principle that it is better to treat disease than to mask it, sedatives should be used only when pain and delirium have been addressed with the use of specific pharmacologic and nonpharmacologic strategies.
PAIN, ANALGESIA, AND SEDATION IN THE ICU
Prospective studies confirm that the majority of patients who are treated in ICUs have pain,1 which makes the assessment of pain and provision of adequate analgesia essential components of ICU care. The short-term consequences of untreated pain include higher energy expenditure and immunomodulation.2,3 Longer-term, untreated pain increases the risk of post-traumatic stress disorder.4 Assessing whether a patient in the ICU is in pain may be difficult. The reference standard for the assessment of pain is self-reporting by the patient, but patients in the ICU may not be sufficiently interactive to give valid responses. Physiological indicators such as hypertension and tachycardia correlate poorly with more intuitively valid measures of pain,5 but pain scales such as the Behavioral Pain Scale6 and the Critical Care Pain Observation Tool7 provide structured and repeatable assessments and are currently the best available methods for assessing pain.
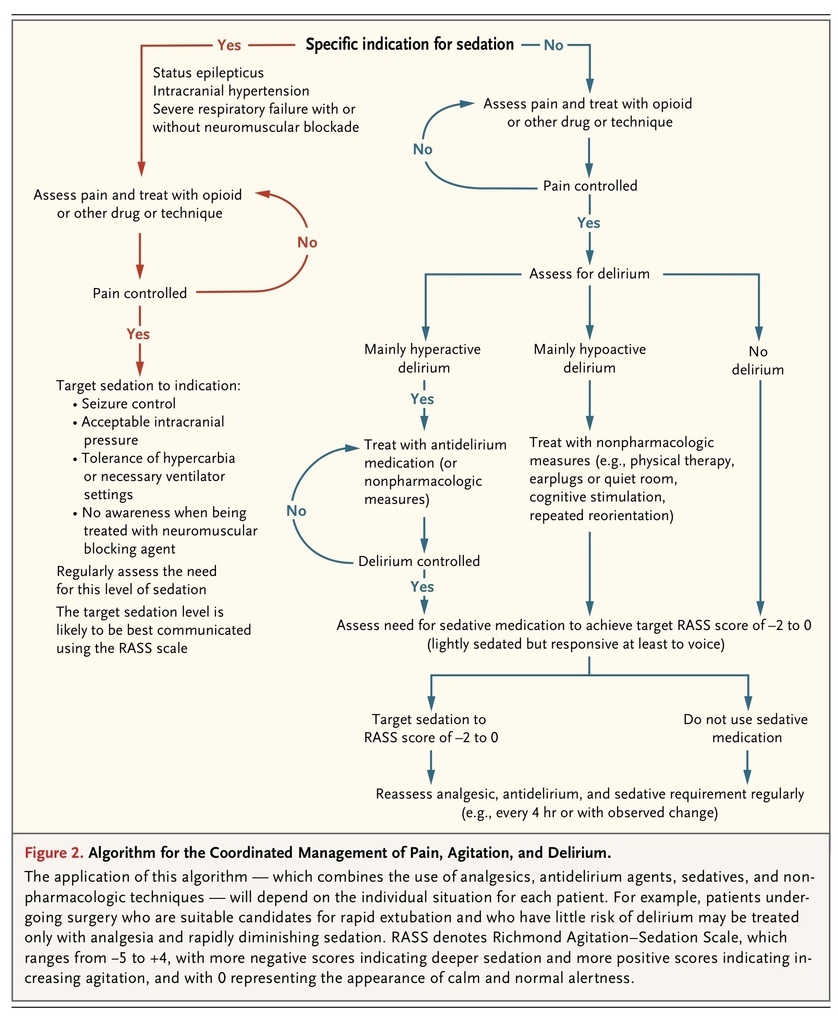
A minority of ICU patients have an indication for continuous deep sedation, for reasons such as the treatment of intracranial hypertension, severe respiratory failure, refractory status epilepticus, and prevention of awareness in patients treated with neuromuscular blocking agents. This review will focus on the remaining overwhelming majority of patients undergoing mechanical ventilation for whom the use of sedatives and analgesics should be minimized, with the goal that they be calm, lucid, pain-free, interactive, and cooperative with their care.
Evidence from randomized, controlled trials consistently supports the use of the minimum possible level of sedation. In a landmark trial that compared routine daily interruption of sedative infusions with discretionary interruption by treating clinicians, patients whose sedation was routinely interrupted received less sedation overall and spent fewer days undergoing mechanical ventilation and fewer days in the ICU. 8 Although the trial was too small to assess differences in mortality or discharge destination, the observed reductions in the duration of mechanical ventilation and length of stay in the ICU were associated with a nonsignificant reduction in mortality and a nonsignificant increase in the proportion of patients who were discharged to their own homes.8 A subsequent larger multicenter trial combined the daily interruption of sedation with daily spontaneous breathing trials.9 Daily interruption of sedation was associated with reduced administration of a benzodiazepine sedative, reduced duration of mechanical ventilation, reduced length of stay in the ICU, and significantly increased survival. In contrast, when daily interruption of sedation was added to a protocol for sedation practice that already sought to minimize the level of sedation, the total sedative dose was increased and there was no clinical benefit.10
These conflicting results are open to a number of interpretations, including that daily interruption is beneficial only when it results in a reduction in the total dose of sedative administered. The conflicting findings also highlight that the results of daily interruption of sedation may be context-specific and will depend on the population being studied, protocol adherence, and management of the control group. A randomized, controlled trial in which all patients undergoing mechanical ventilation received morphine for the treatment of pain in an “analgesia first” approach compared a protocol of no sedation with the routine use of sedation with daily interruption.11 Patients who were assigned to the protocol of no sedation had shorter stays in the ICU and the hospital and more days without mechanical ventilation.
The consistent message from all these sedation-interruption trials is that minimizing sedation among patients in the ICU provides clinical benefit. Further support comes from a prospective, multicenter, longitudinal cohort study showing that the depth of sedation was independently associated with the duration of mechanical ventilation, in-hospital mortality, and rates of death within 180 days.12 In a randomized, controlled trial, the use of lighter sedation resulted in more ventilator-free and ICU-free days.13 In comparison with deep sedation, the use of lighter sedation did not increase the rate of short-term adverse events, and long-term psychiatric outcomes were either unaffected or improved. 13-16
CHOICE OF SEDATIVE AGENT
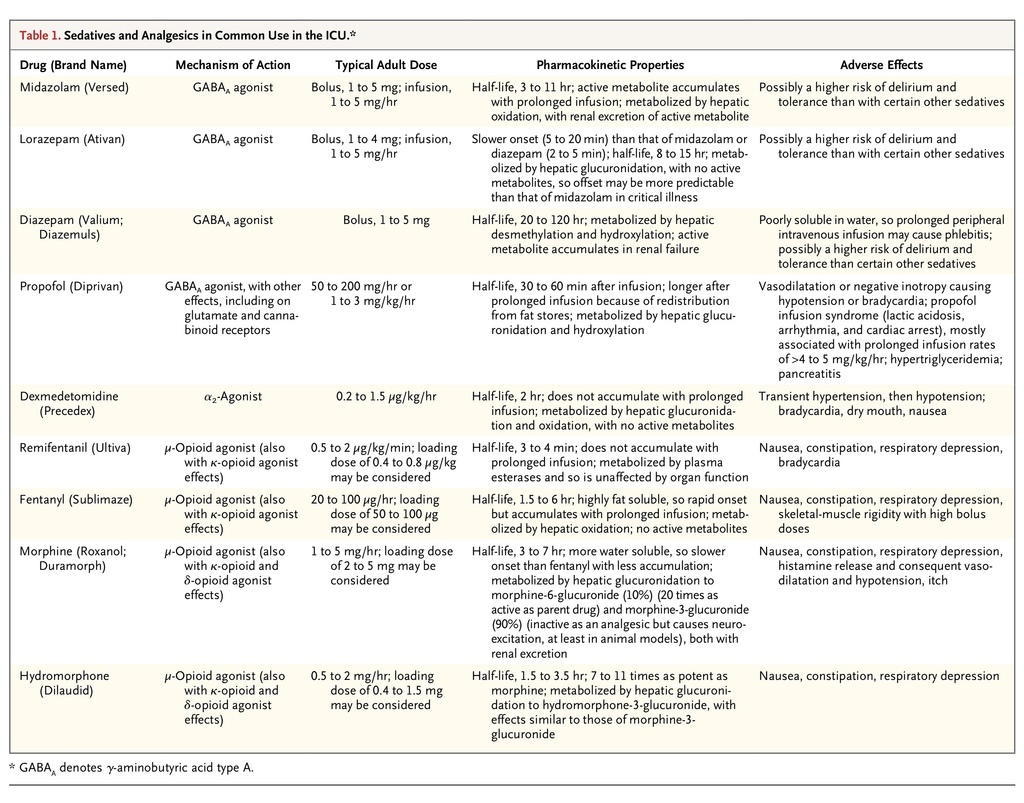
Despite at least 90 trials comparing sedative regimens,17 in general, no sedative drug is clearly superior to all others. Sedatives that are commonly used in the ICU are the benzodiazepines midazolam and lorazepam (and to a lesser extent, diazepam), the short-acting intravenous anesthetic agent propofol, and dexmedetomidine.12 Remifentanil, an opioid, is also used as a sole agent because of its sedative effects. Benzodiazepines act through γ-aminobutyric acid type A (GABAA) receptors, as in part does propofol, whereas dexmedetomidine is an α2-adrenoceptor agonist, and remifentanil is a μ-opioid receptor agonist (Table 1
TABLE 1
Sedatives and Analgesics in Common Use in the ICU.
). Marked differences in prescribing patterns among countries suggest that the choice of agent is determined more by tradition and familiarity than by evidence-based practice.
If minimizing the depth and duration of sedation is accepted as a desirable goal, then the use of a short-acting agent with an effect that can be rapidly adjusted such as propofol or remifentanil should offer advantages over longer-acting agents or agents with active metabolites. As compared with benzodiazepines, propofol has not been shown to reduce mortality but may result in a reduction in the length of stay in the ICU.18 Dexmedetomidine may also have advantages over benzodiazepines, since it produces analgesia, causes less respiratory depression, and seemingly provides a qualitatively different type of sedation in which patients are more interactive and so potentially better able to communicate their needs.19 As compared with lorazepam and midazolam, dexmedetomidine resulted in less delirium and a shorter duration of mechanical ventilation but not reduced stays in the ICU or hospital. 19-21 When two short-acting and titratable drugs such as propofol and dexmedetomidine were compared, there was no significant difference in the time spent at the target sedation level and no difference in either the duration of mechanical ventilation or ICU stay.19
Remifentanil has a half-life of 3 to 4 minutes that is independent of the infusion duration or organ function. It has been investigated as a sedative agent in ICUs predominantly among surgical patients. It has been compared with midazolam alone, midazolam with fentanyl, fentanyl alone, and morphine.22-25 Although remifentanil has been associated with a reduced duration of mechanical ventilation and ICU stay in these small trials, it has not yet been evaluated in a large, heterogeneous population of critically ill patients and is currently not a common choice in most ICUs.
PREVENTION AND TREATMENT OF DELIRIUM
The Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV),26 lists four domains of delirium: disturbance of consciousness, change in cognition, development over a short period, and fluctuation. Delirium is defined by the National Institutes of Health as “sudden severe confusion and rapid changes in brain function that occur with physical or mental illness.” The most common feature of delirium, thought by many to be its cardinal sign, is inattention. Delirium is a nonspecific but generally reversible manifestation of acute illness that appears to have many causes, including recovery from a sedated or oversedated state.
The pathophysiology of delirium that is associated with critical illness remains largely uncharacterized and may vary depending on the cause. The increased risk associated with the use of GABAA agonists and anticholinergic drugs led to the suggestion that the GABAergic and cholinergic neurotransmitter systems play a contributory role. In particular, central cholinergic deficiency may be a final common pathway. Alternative hypotheses include excess dopaminergic activity and direct neurotoxic effects of inflammatory cytokines. Currently, these hypotheses are unproven, making pharmacologic management strategies largely empirical.
Studies using magnetic resonance imaging have shown a positive association between the duration of delirium in the ICU and both cerebral atrophy and cerebral white-matter disruption.27,28 These preliminary investigations indicate either that delirium in the ICU gives rise to alterations in brain structure or that the presence of such cerebral atrophy and white-matter disruption renders patients more susceptible to delirium.
Regardless of the cause and the underlying pathophysiology, delirium is now recognized as a frequent and serious event in critically ill patients. There is no diagnostic blood, electrophysiological, or imaging test for delirium, which therefore remains a clinical diagnosis. Estimates for the incidence of delirium in the ICU range from 16%29 to 89%,30 with the reported incidence affected both by the characteristics of the population being studied and by the diagnostic criteria used. Risk factors that have been identified include an advanced age and the presence of more than one condition associated with coma, followed by treatment with sedative medications, a neurologic diagnosis, and increased severity of illness.31 A diagnosis of delirium is associated with increased mortality (estimated as a 10% increase in the relative risk of death for each day of delirium32) and decreased long-term cognitive function. 33
There are two distinct forms of delirium, hypoactive and agitated (or hyperactive). When individual patients intermittently have both forms, it is termed mixed delirium. The hypoactive form is characterized by inattention, disordered thinking, and a decreased level of consciousness without agitation. Pure agitated delirium affects less than 2% of patients with delirium in the ICU.34 Patients with hypoactive delirium are the least likely to survive, but those who do survive may have better long-term function than those with agitated or mixed delirium.33 Separating the effects of delirium status from those of illness severity with respect to the risk of death is difficult, since patients with more serious illnesses are at increased risk for both delirium and death. Association studies typically adjust for illness severity on admission to the ICU rather than at the time that delirium is diagnosed. Although the association between delirium and a worse outcome is clear, a causal relationship has not been established. Currently, the evidence that specific treatment of delirium may improve outcomes is tenuous.
ASSESSMENT AND MONITORING OF SEDATION AND DELIRIUM
Although ICU practice is characterized by close monitoring of carefully administered care, surveys that have been conducted in various countries have shown that the depth of sedation frequently goes unmonitored.35 This finding is surprising and unacceptable, since evidence suggests that the routine monitoring of sedation may improve patients' outcomes.36
Sedation Scales
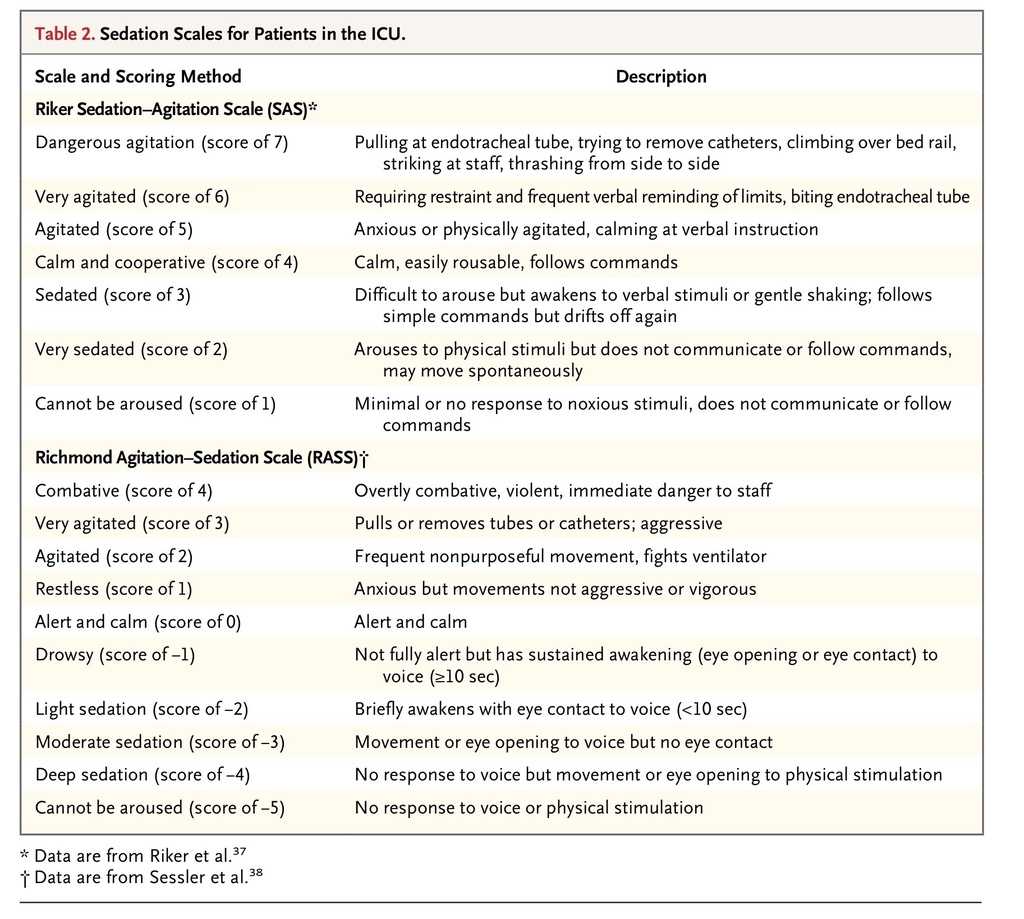
Of the sedation scales described, the Riker Sedation–Agitation Scale37 and the Richmond Agitation–Sedation Scale38 are the most commonly reported, but in head-to-head comparison, neither is demonstrably superior39 (Table 2
TABLE 2
Sedation Scales for Patients in the ICU.
). For the majority of patients undergoing mechanical ventilation in an ICU, an appropriate target is a score of 3 to 4 on the Riker Sedation–Agitation Scale (which ranges from 1 to 7, with scores of <4 indicating deeper sedation, a score of 4 indicating an appearance of calm and cooperativeness, and scores of ≥5 indicating increasing agitation) or a score of −2 to 0 on the Richmond Agitation–Sedation Scale (which ranges from −5 to +4, with more negative scores indicating deeper sedation and more positive scores indicating increasing agitation, and with 0 representing the appearance of calm and normal alertness).
Identifying Delirium
In routine practice, ICU staff members typically do not diagnose delirium in almost three quarters of their patients who have the condition, whereas active screening by research nurses identified delirium in up to 64% of patients who were considered to be delirious by a psychiatrist, a geriatrician, or a neurologist.40 Scales with respect to delirium in the ICU apply the four DSM-IV domains defining delirium in general medical and psychiatric patients to those in the ICU whose severity of illness can rapidly fluctuate, who receive multiple analgesics and sedatives, and who are unable to speak owing to endotracheal intubation. Two scales are in common use, the Confusion Assessment Method for the ICU (CAM-ICU)41 and the Intensive Care Delirium Screening Checklist (ICDSC)29 (Table 3
TABLE 3
Scoring Systems for the Diagnosis of Delirium in Critically Ill Patients.
). The CAM-ICU reports a dichotomous assessment at a single time point, whereas the ICDSC lists signs that can be observed over a period of time. Although such scales are essential in objectively diagnosing delirium for research purposes, it is not clear that the use of these scales is more sensitive than unstructured assessments made by trained bedside nurses who are prompted to look for delirium. Some studies have shown a high sensitivity when such assessments are performed by bedside nurses,42 whereas other studies have shown conflicting results.43 Used alone (without an accompanying sedation scale), none of the published scales distinguish hyperactive from hypoactive delirium, and none of the published scales quantify the relative importance of individual elements of the scales despite recognition that specific treatments may shorten the duration of some elements and prolong the duration of others. All the scales dichotomize delirium as being either present or absent, although it would seem to be intuitive that delirium has different degrees of severity. The CAM-ICU and ICDSC are currently the two accepted methods for identifying a condition that otherwise frequently goes undiagnosed.44
PREVENTION AND TREATMENTOF DELIRIUM
Prevention
There is some evidence that delirium can be prevented. Outside the ICU, repeated reorientation, noise reduction, cognitive stimulation, vision and hearing aids, adequate hydration, and early mobilization can reduce the incidence of delirium in hospitalized patients.45 Haloperidol prophylaxis in patients undergoing hip surgery reduced the severity and duration of delirium.46 Among patients in the ICU, the duration of delirium was cut in half with early mobilization during interruptions in sedation.47
Pharmacologic studies of delirium prevention include trials comparing one sedative–analgesic regimen with another and studies of antipsychotic drugs administered with the specific intent of preventing delirium. Four placebo-controlled trials have evaluated pharmacologic prophylaxis of delirium; low-dose haloperidol48 and low-dose risperidone49 both reduced the incidence of delirium, as did a single low dose of ketamine during the induction of anesthesia.50 However, these trials were conducted among patients undergoing elective surgical procedures, and it is not clear whether their results can be extrapolated to the general ICU population. In contrast, the cholinesterase inhibitor rivastigmine was ineffective in preventing delirium.51
Sedation with dexmedetomidine rather than benzodiazepines appears to reduce the incidence of delirium in the ICU. In a multicenter, randomized trial predominantly involving medical patients in the ICU, the administration of dexmedetomidine or midazolam resulted in similar proportions of time within the target range of −2 to +1 on the Richmond Agitation–Sedation Scale among patients, but those assigned to receive dexmedetomidine had a reduced risk of delirium and spent less time undergoing mechanical ventilation.21 As compared with a lorazepam infusion, sedation with dexmedetomidine resulted in more time at the target level of sedation and longer survival without delirium or coma.20 In a multicenter European trial, patients were randomly assigned to continue treatment with their current sedative (midazolam or propofol) or to switch to sedation with up to 1.4 μg of dexmedetomidine per kilogram of body weight per hour.19 There were no between-group differences in the proportion of time at the target level of sedation. The rates of the composite end point of agitation, anxiety, or delirium were lower with dexmedetomidine than with propofol, but the rates with dexmedetomidine were equivalent to those with midazolam. When delirium was assessed with the use of the CAM-ICU 48 hours after sedation was discontinued, there were no significant differences among the groups.
Treatment
There is very little evidence to guide the management of established delirium, and most existing trials were categorized by the investigators as pilot studies. Only one small placebo-controlled trial supports the efficacy of a drug treatment for established delirium in patients in the ICU. In a study of 36 patients who were randomly assigned to treatment with quetiapine or placebo, delirium resolved faster in patients who received quetiapine. The use of quetiapine also increased the number of patients who were discharged to their own home or to rehabilitation.52 A study of 103 patients who were randomly assigned to receive regular haloperidol, ziprasidone, or placebo showed no significant differences in the number of days that patients survived without delirium or coma.53 The single study comparing haloperidol with an atypical antipsychotic (olanzapine) showed equivalent efficacy.54 None of these trials distinguished between hyperactive and hypoactive delirium.
In a pilot study comparing dexmedetomidine with haloperidol in patients with hyperactive delirium, dexmedetomidine was associated with a shorter time to extubation and shorter length of stay in the ICU.55 This finding is supported by a randomized trial of dexmedetomidine versus midazolam in which patients with delirium at the time of enrollment had a more rapid resolution of delirium if they were assigned to receive dexmedetomidine than if they were assigned to receive midazolam.21 However, definitive evidence supporting the use of dexmedetomidine for the treatment of delirium is not currently available.
QUALITY IMPROVEMENT TECHNIQUES
Frameworks that facilitate the aforementioned approaches have been developed. These include the “pain, agitation, and delirium” (PAD) guidelines44 and the “spontaneous awakening and breathing coordination, attention to the choice of sedation, delirium monitoring, and early mobility and exercise” (ABCDE) bundle.56 These guidelines emphasize improving team communication in the ICU, standardizing care processes, and prioritizing methods to lighten sedation and facilitate early mobilization and extubation. Each guideline recognizes the conceptual evolution from spontaneous-breathing trials and interruption of sedation to a comprehensive approach to monitoring and managing pain, agitation, and delirium.
CONCLUSIONS
Accumulating evidence suggests that the management of sedation and delirium can have an important effect on the outcomes of patients who are treated in ICUs. Currently available data suggest that the best outcomes are achieved with the use of a protocol in which the depth of sedation and the presence of pain and delirium are routinely monitored, pain is treated promptly and effectively, the administration of sedatives is kept to the minimum necessary for the comfort and safety of the patient, and early mobilization is achieved whenever possible.







 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 