CLINICAL CONTEXT
The Centers for Disease Control and Prevention (CDC) and partner organizations annually update and publish recommended immunization schedules for healthy adults and children as well as for certain adults and children at high risk for vaccine-preventable infections. The goal of these new guidelines from the Infectious Diseases Society of America (IDSA) is to reduce morbidity and mortality rates from vaccine-preventable infections in patients who are immunocompromised.
Immunocompromised patients have impaired host defenses predisposing them to greater risk or severity for vaccine-preventable infection, and vaccination of this group is therefore important. In addition, these patients are in frequent contact with medical environments and may therefore also have greater exposure to pathogens. Nonetheless, vaccination rates of this group are often low because clinicians have insufficient or inaccurate information concerning the safety and efficacy of, and contraindication to, vaccination of such patients.
STUDY SYNOPSIS AND PERSPECTIVE
Most immunocompromised people should be vaccinated against influenza and other infections, according to new guidelines from the IDSA published online December 4 in Clinical Infectious Diseases. Because clinicians may be concerned about safety and efficacy, however, vaccination rates tend to be lower in patients with compromised immune systems.
"The guideline provides 'one-stop shopping' for clinicians caring for children and adults with compromised immune systems and includes recommendations and evidence for all vaccinations, from influenza to chicken pox," said lead author Lorry G. Rubin, MD, director of the Division of Pediatric Infectious Diseases at the Steven and Alexandra Cohen Children's Medical Center of New York in New Hyde Park, and professor of pediatrics at Hofstra North Shore-LIJ School of Medicine, in a news release. "Previously, the recommendations were difficult to retrieve because in most cases information had to be accessed individually by vaccine rather than by the category of patient disease."
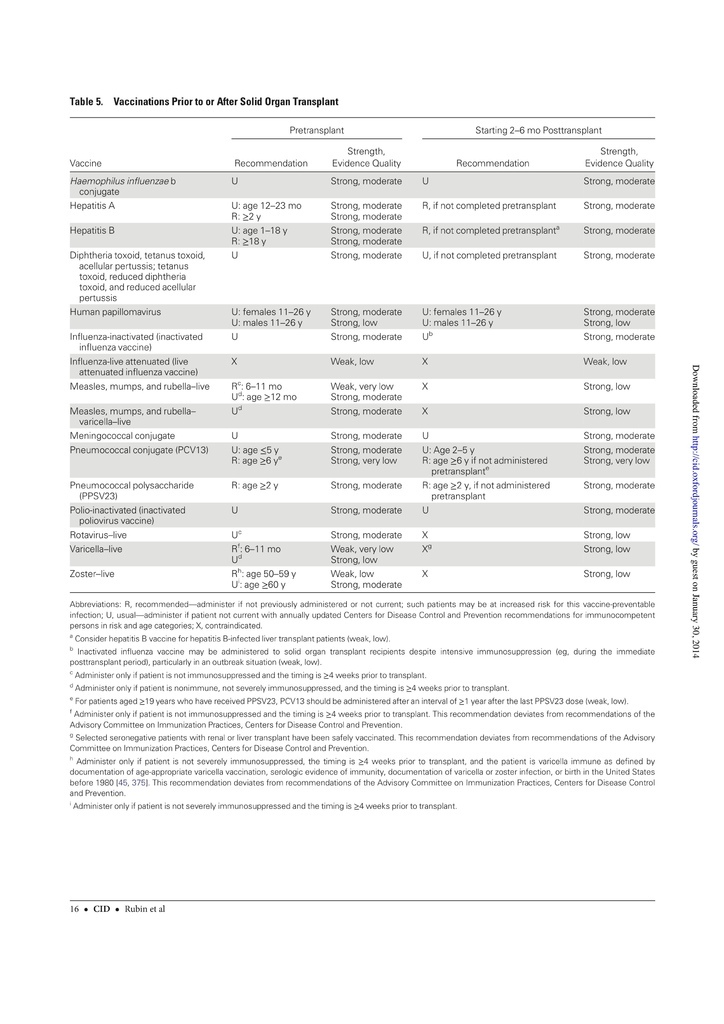
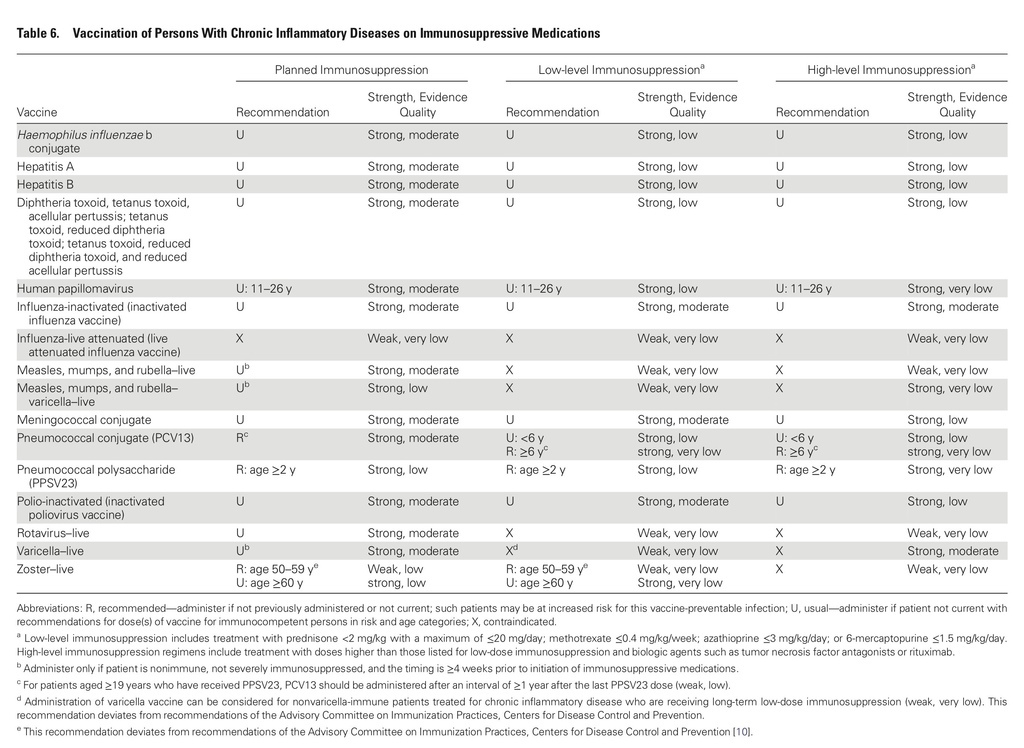
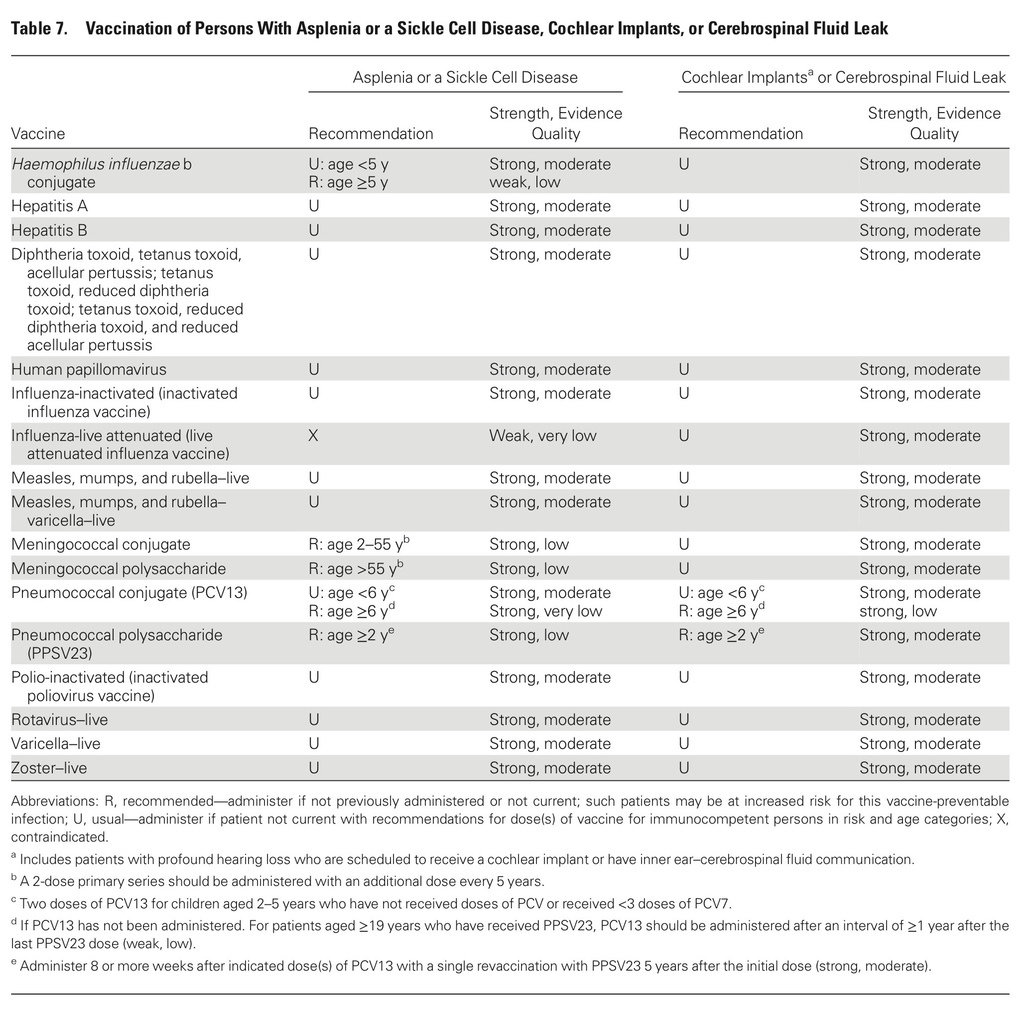
The guidelines target primary care providers and specialists caring for patients with compromised immune systems from HIV infection or AIDS, cancer, solid organ transplantation (eg, kidney or liver), stem cell transplantation, sickle cell disease or asplenia, congenital immune deficiencies, chronic inflammatory conditions (eg, rheumatoid arthritis or Crohn's disease), cochlear implants, or cerebrospinal fluid leaks.
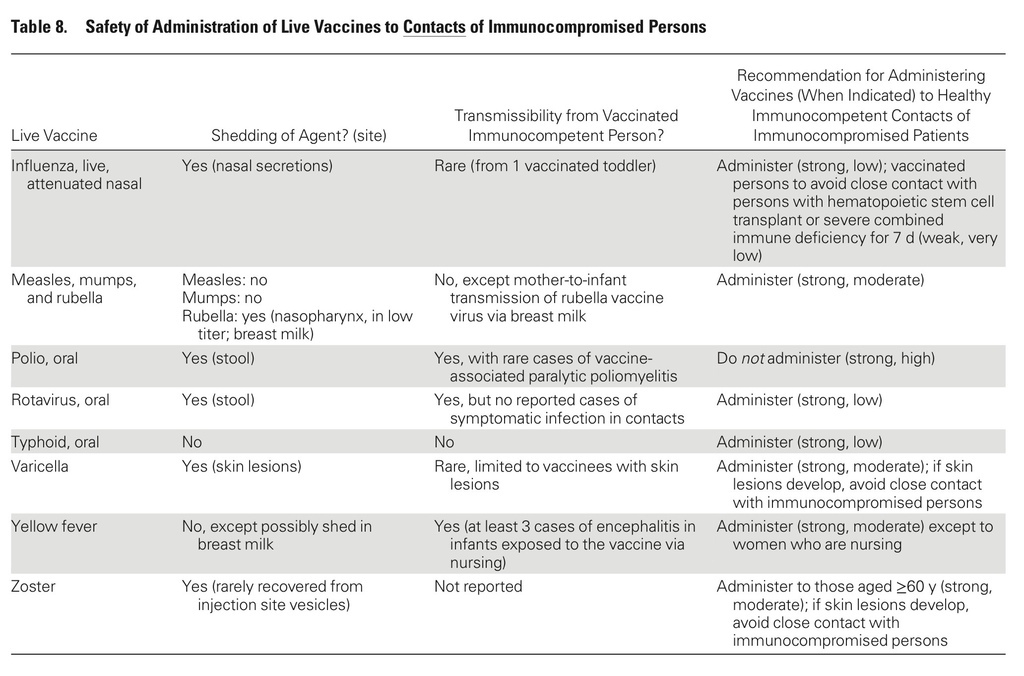
In addition, the guidelines include recommendations for vaccination of people living with immunocompromised patients.
Recommendations cover most available vaccines, including hepatitis A, measles, mumps and rubella, other childhood vaccinations, and those for pneumococcus and herpes zoster.
Specific recommendations include the following:
- If feasible, vaccines should be given before planned immunosuppression.
- Live vaccines should be given at least 4 weeks before immunosuppression and should be avoided within 2 weeks of initiation of immunosuppression.
- Inactivated vaccines should be given at least 2 weeks before immunosuppression.
- Most immunocompromised patients at least 6 months old should receive annual influenza vaccination as an injection, but they should not receive live attenuated influenza vaccine (LAIV) administered as a nasal spray.
- Influenza vaccine is unlikely to benefit patients receiving intensive chemotherapy or who have received anti-B-cell antibodies in the previous 6 months.
- Immunocompetent persons living in a household with immunocompromised patients can safely receive inactivated vaccines based on the CDC-Advisory Committee on Immunization Practices (ACIP) annually updated recommended vaccination schedules for children and adults or for travel.
- Persons living in a household with immunocompromised patients at least 6 months old should annually receive influenza vaccine, either inactivated influenza vaccine (IIV), or LAIV provided they are healthy, not pregnant, and between 2 and 49 years old.
An expert panel developed these guidelines, which are based on limited evidence. Experts included adult and pediatric specialists in gastroenterology, immunology, infectious diseases, hematology, oncology, rheumatology, and stem-cell and solid-organ transplantation.
Mobile device and pocket-sized quick-reference editions of the guidelines and other guideline-related products are available on the IDSA Web site.
The IDSA provided support for this guideline. Some of the guideline authors have disclosed various financial relationships with ViroPharma; Roche; the CDC; Abbott; UCB; Merck; Dyax; Cubist; Nutricia; Up-To-Date, Inc; GlaxoSmithKline; Pfizer; Amino Up Chemical; MedImmune; Sanofi Pasteur; Vical; Clinigen; Astellas Pharma; and/or AiCuris.
Clin Infect Dis. Published online December 4, 2013.
STUDY HIGHLIGHTS
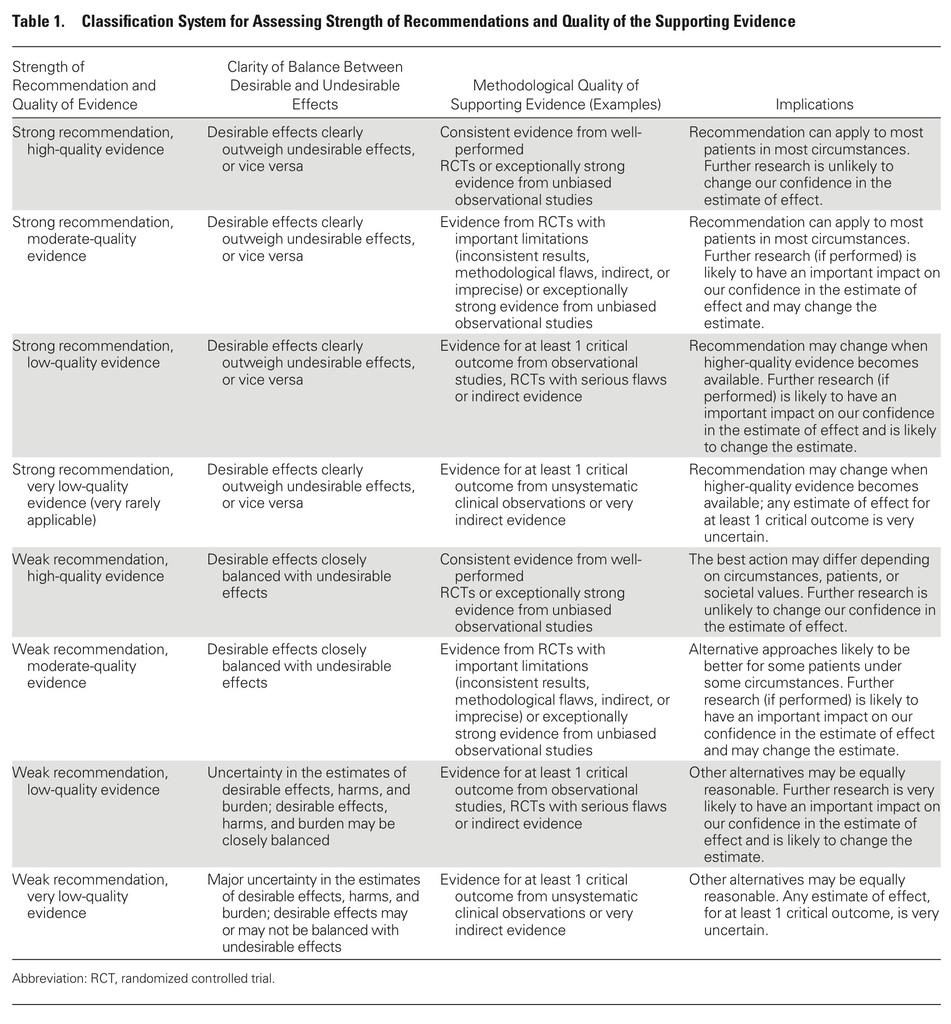
- The decision to give or to withhold a vaccine should be based on balancing the burden of the vaccine-preventable disease and risk for the development of severe or life-threatening infection with the risk for adverse effects from vaccination.
- Few data are available on vaccine efficacy in immunocompromised patients.
- Most clinical evidence indicates that vaccines are not important triggers of disease flares in immunocompromised patients or of rejection in solid organ transplant recipients. Therefore, vaccines should not be withheld for these reasons.
- Specialists who care for immunocompromised patients share responsibility with the primary care provider for ensuring that appropriate vaccinations are administered to immunocompromised patients and to members of their households.
- Patients with primary complement deficiencies should receive all routine vaccines based on the CDC annual schedule; none are contraindicated.
- Patients with suspected combined immunodeficiencies can be given all inactivated vaccines as part of immune response assessment before starting immunoglobulin therapy.
- Once these patients begin immunoglobulin therapy, however, they should not routinely be given inactivated vaccines.
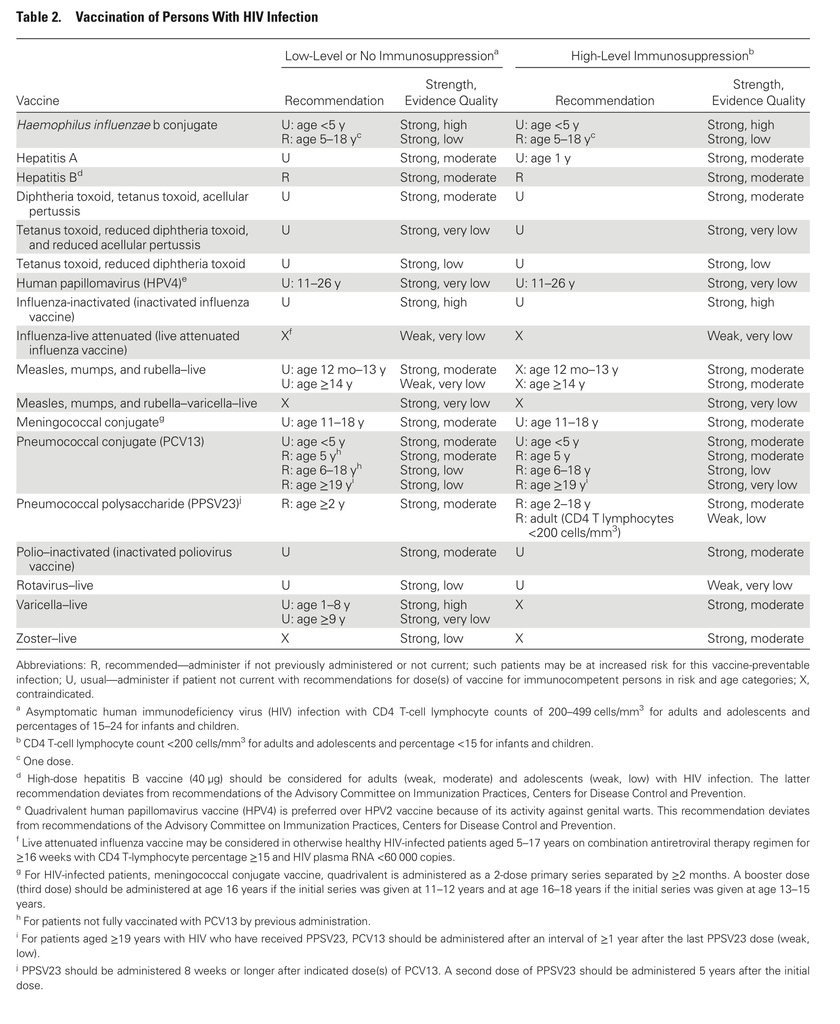
- HIV-infected patients should be vaccinated according to the CDC annual schedule for most inactivated vaccines, but they should not receive LAIV.
- HIV-exposed or HIV-infected infants should receive rotavirus vaccine according to the schedule for uninfected infants.
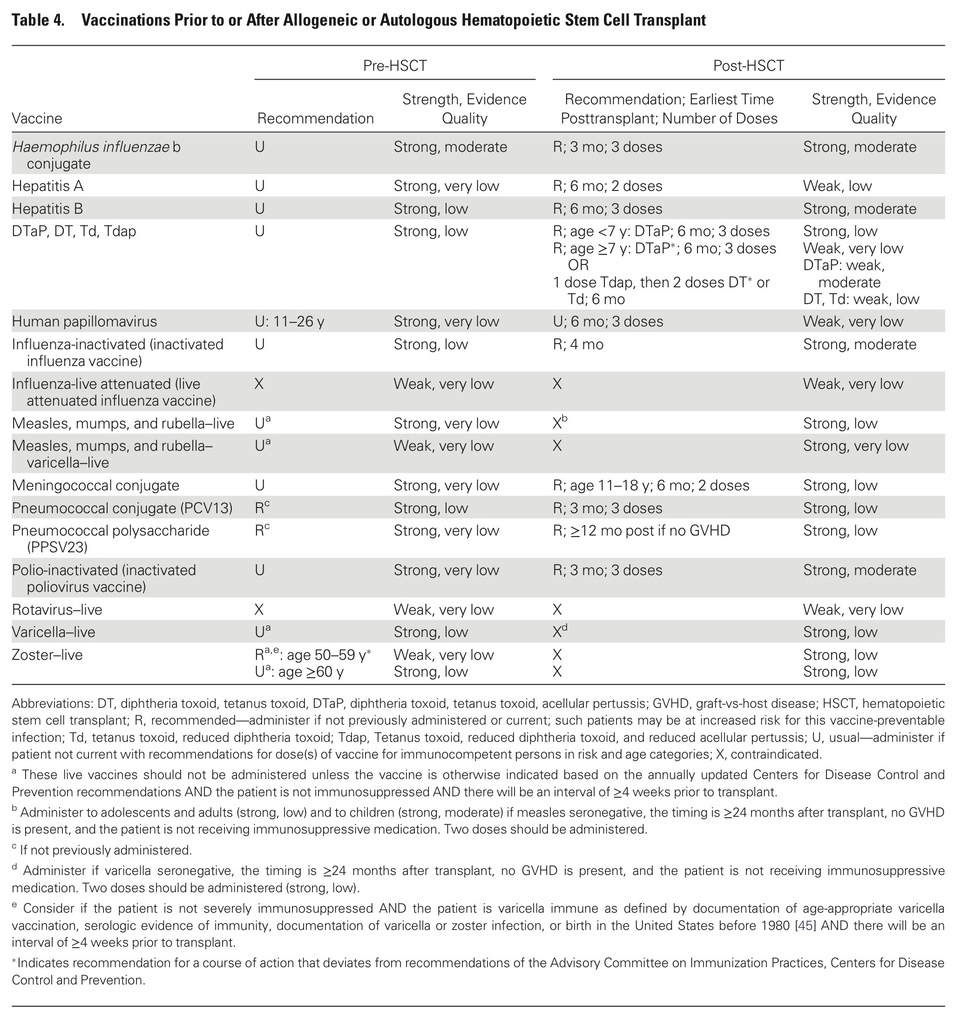
- Patients should undergo vaccination before planned immunosuppression, whenever feasible.
- Live vaccines should be given at least 4 weeks before immunosuppression and should be avoided within 2 weeks of initiation of immunosuppression.
- Inactivated vaccines should be given at least 2 weeks before immunosuppression.
- It is safe to give inactivated vaccines to immunocompetent persons who reside with immunocompromised patients, using the CDC-ACIP's annually updated recommended vaccination schedules for children and adults or for travel.
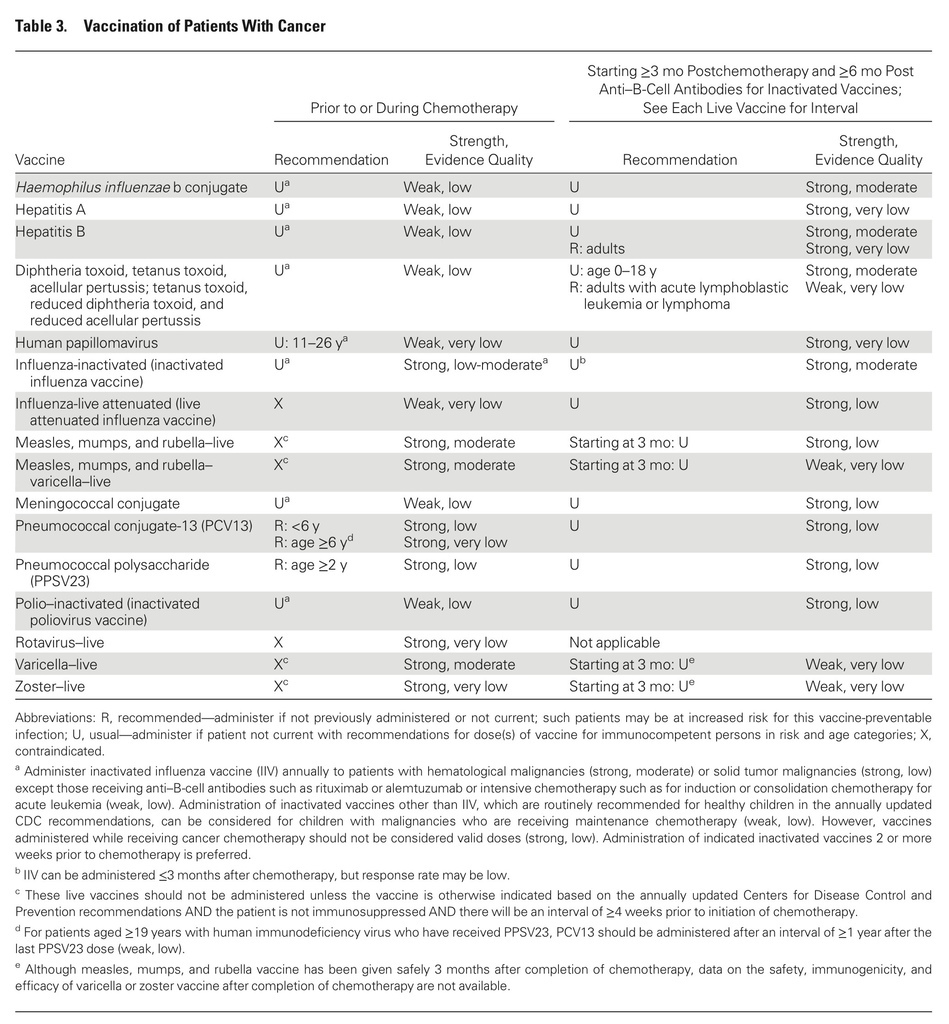
- IIV should be administered annually as an injection to most immunocompromised patients at least 6 months old, including those with hematologic or solid tumor malignancies.
- Immunocompromised patients should not receive LAIV given as a nasal spray.
- Patients receiving intensive chemotherapy or who were given anti-B-cell antibodies in the previous 6 months are unlikely to benefit from influenza vaccine.
- Influenza vaccine should be given annually to persons living in a household with immunocompromised patients at least 6 months old.
- This may be either IIV, or LAIV if they are healthy, not pregnant, and between 2 and 49 years old.
CLINICAL IMPLICATIONS
- Deciding whether to give or to withhold a vaccine from an immunocompromised patient involves weighing the burden of the vaccine-preventable disease and risk for the development of severe or life-threatening infection with the risk for adverse effects from vaccination. New IDSA guidelines offer detailed recommendations for specific immunocompromising conditions and vaccinations, but the evidence base is limited.
- The IDSA guidelines recommend that IIV be given annually to most immunocompromised patients at least 6 months old, but LAIV should be avoided in these patients.






 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 