Abscesses are one of the most common skin conditions managed by general practitioners and emergency physicians. The incidence of skin abscesses has increased,1-5 and this increase has coincided with the emergence of community-associated methicillin-resistant Staphylococcus aureus (MRSA). In many parts of the world, MRSA infections are now the most common cause of skin abscesses.6 Community-associated MRSA has also been found to cause severe infections — including necrotizing pneumonia, necrotizing fasciitis, purpura fulminans, and severe sepsis — in nonimmunocompromised hosts; however, its apparently increased virulence as compared with that of health care–associated strains and methicillin-susceptible S. aureus is incompletely understood.7-10 Along with the increases in the incidence of skin abscesses and MRSA infections, other changes that potentially affect abscess care have occurred. Bedside ultrasonography has become increasingly available in emergency departments and hospitals. Traditional surgical practices have been systematically tested, and new techniques developed. Prevention strategies have also been investigated. Despite these changes, the management of skin abscesses is highly variable.11-13
In this article, we describe our approach to the management of common skin abscesses that generally involve the extremities and trunk. Lesions that may require unique surgical approaches or that have a more complex microbiologic basis, such as abscesses in the perineal area, are not addressed. Whenever possible, our recommendations are based on randomized trials. However, many of the recommendations are based on small, observational studies or expert opinion; thus, we recognize that there may be disagreement with some of our recommendations. Nevertheless, the approach we advise has been shown to be workable and useful in our practice.
DIAGNOSIS
A skin abscess results from the accumulation of pus in the dermis or subcutaneous tissue and appears as a swollen, red, tender, and fluctuant mass, often with surrounding cellulitis. Diagnosis of a skin abscess based on physical examination is often straightforward and proved correct by incision and drainage. However, abscesses that extend deeper into the dermis and subcutaneous tissue, especially those associated with extensive cellulitis, may be more difficult to diagnose because overlying tissue induration may prevent fluctuance from being observed. Physicians' clinical assessments during physical examination also vary. In one study, involving 349 children presenting to a hospital with skin and soft-tissue infections, interobserver agreement among pediatric attending physicians and fellows regarding the presence of an abscess was only fair and was not associated with the extent of the physician's experience.14
Studies in adults and children suggest that soft-tissue ultrasonography enhances the diagnostic accuracy of abscess detection and alters plans for management that are based on physical examination alone. In a prospective study involving 126 adults with clinical cellulitis in whom an emergency physician believed an abscess was not obvious on physical examination but might be present, ultrasonography resulted in a change in projected management in 56% of the patients. 15 Ultrasonographic images showed fluid collection that was consistent with an abscess in half these patients, and approximately 80% of patients who underwent additional diagnostic testing had pus or other fluid collections. Management was also altered in three quarters of patients in whom drainage had been thought to be required on the basis of physical examination alone (e.g., it was decided that drainage was not needed, that further imaging was required, or that the incision and drainage approach should be altered). A study involving children with signs and symptoms of skin and soft-tissue infection showed that for the detection of abscesses, ultrasonography was significantly more sensitive than and about as specific as physical examination, with management changed on the basis of the ultrasonographic findings in approximately 14% of patients.16 In another pediatric study, ultrasonography improved diagnostic accuracy only when clinical examination did not clearly indicate the presence of a skin lesion requiring drainage.17
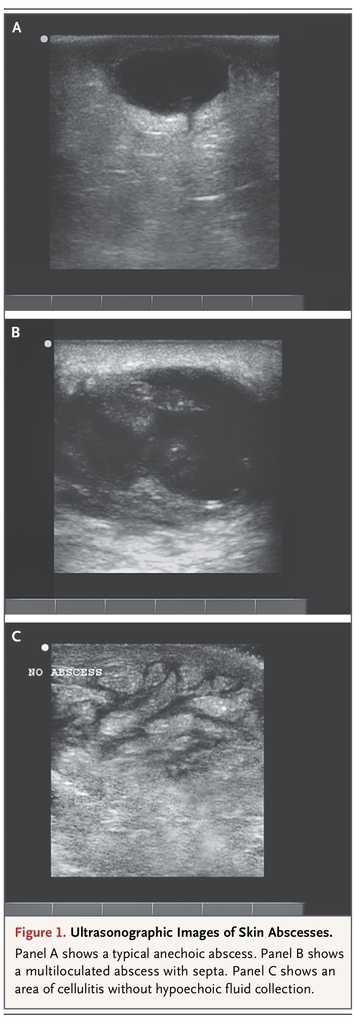
In addition, ultrasonography performed before and after fluid evacuation is helpful in ensuring the adequacy of drainage. Ultrasonography performed by a physician experienced in its use should be considered for large and indurated areas of apparent cellulitis in which the clinician believes that physical examination cannot rule out a deep abscess, particularly for patients treated for cellulitis in whom initial antibiotic treatment fails. Ultrasonographic images of cutaneous abscesses are shown in Figure 1
FIGURE 1
Ultrasonographic Images of Skin Abscesses.
. Mature abscesses have a dark, anechoic or hypoechoic, fluid-filled center, whereas abscesses at an earlier stage of development may be harder to recognize, with a collection of isoechoic material that is somewhat distinct in appearance from the surrounding tissue.
Needle aspiration has been used for both diagnosing and treating abscesses. Although aspiration of pus can confirm the presence of an abscess, an absence of aspirated purulent material does not necessarily rule out the presence of an abscess.18 Furthermore, needle aspiration may not be adequate to drain all the pus, possibly because of the high viscosity and fibrin content of pus in staphylococcal abscesses.19
TREATMENT
Drainage
Many abscesses can be managed in the office setting by a general practitioner. Large, complex, or recalcitrant abscesses, especially those over sensitive areas (e.g., the hands or face), should prompt consideration of referral to a specialist or an emergency department, where additional resources can be brought to bear. The primary treatment for skin abscesses is incision and drainage.20 Readers are referred to the Video in Clinical Medicine that explains the standard techniques for drainage of an abscess (www.nejm.org/doi/full/10.1056/NEJMvcm071319).21 A single incision is made; the incision should be long enough to ensure complete drainage, allow lysis of loculations with a blunt instrument, and follow tension lines in order to minimize scarring. A common mistake is to make an incision that is not deep enough to reach and fully drain the abscess cavity. Particular care should be taken before incising the skin over critical structures, such as major vessels and nerves. A recent small study among adults suggested that many abscesses can be adequately drained through a short incision (median length, 1 cm).22 We are not aware of trials comparing the effect of opening some versus all loculations. For small lesions (i.e., <2 cm) suggestive of an abscess, such as those that have central induration and pointing but are not clearly fluctuant, one acceptable treatment option is the application of local heat with close follow-up. Systemic antibiotics should be given to patients with systemic signs of infection.
In a study of 15 common emergency-department procedures, abscess drainage was rated as the second most painful procedure, after nasogastric intubation.23 Although local or regional anesthesia may be adequate, procedural sedation or general anesthesia should be considered for patients with very large abscesses, for children, and for patients with abscesses located over particularly sensitive areas.
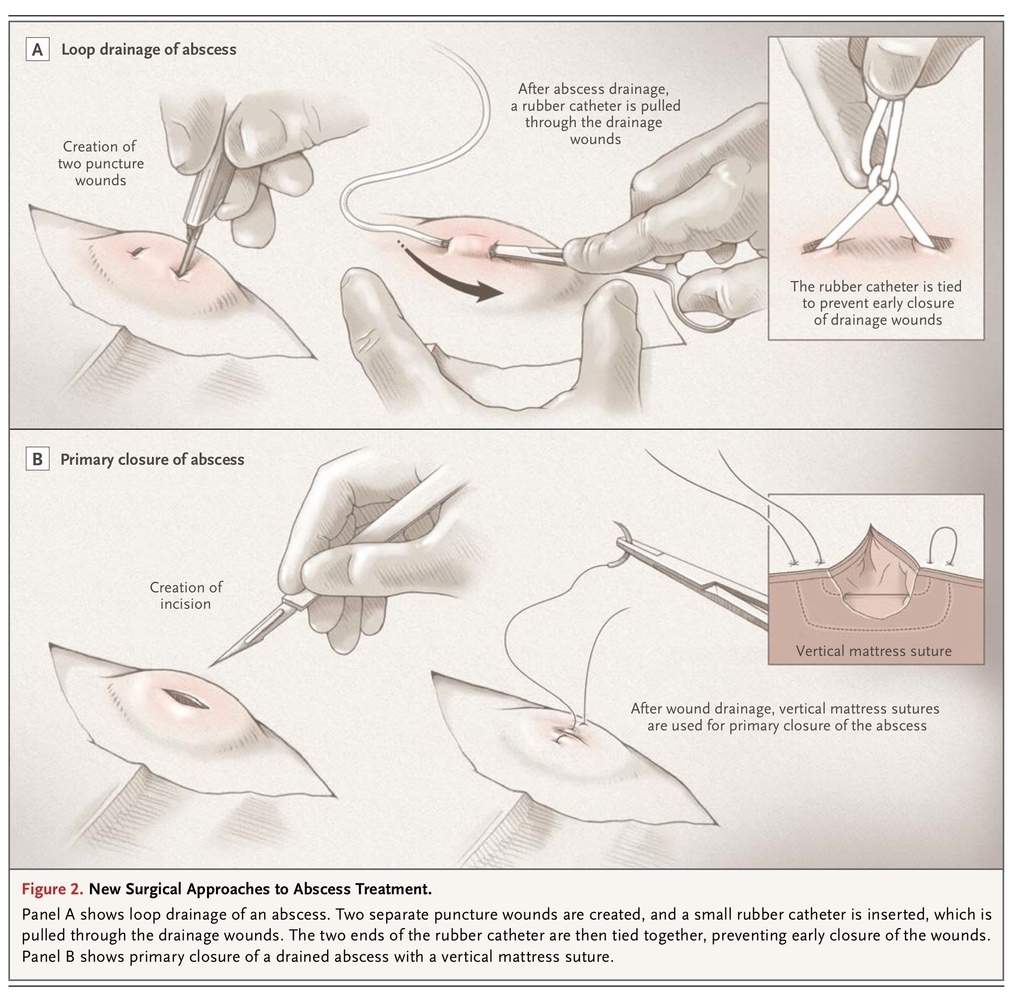
Alternative drainage techniques have been investigated. In a randomized trial involving 101 patients with an abscess who were seen in an emergency department, treatment success, defined as ultrasound-documented complete drainage and symptom resolution by day 7, was achieved significantly less frequently with ultrasound-guided needle aspiration than with incision and drainage — in 26% versus 80% of patients.18 Thus, if needle aspiration is attempted and is initially successful, the patient should be advised of the possible need for later incision and drainage if the infection fails to respond. Making two stab incisions (e.g., 4 to 5 mm each) through which loculations are opened and irrigation performed, with drains placed through the incisions, has been proposed as a less painful approach for children that averts the need for packing. A retrospective study examined drain placement through the abscess cavity with the drain tied in a loop, as compared with open incision and drainage with daily packing, in the treatment of children; the study showed that loop drainage was as safe and efficacious as open incision and drainage and led to better cosmetic results (Figure 2
FIGURE 2
New Surgical Approaches to Abscess Treatment.
).24 Another study described a similar approach in 115 children.25 However, in our experience, most abscesses can be drained with a single small incision.
Irrigation and Packing
The benefit of routine irrigation of the abscess cavity has not been studied, but the need for packing after standard incision and drainage has been investigated. One randomized trial, comparing outcomes of abscess treatment with and without packing in 48 adults who were seen in an emergency department, showed that packing was associated with more pain but similar healing and failure rates, as compared with no packing.26 In another trial, involving 57 children seen in an emergency department, children in the group without packing had higher recurrence rates at 1 month and more frequently required subsequent drainage and antibiotic treatment; however, these differences were not significant, and pain scores in the group without packing were similar to those in the group with packing.27 These studies may have been underpowered to detect clinically significant differences in outcome. For very large abscesses, use of a wick or drain may be considered in place of packing.
Primary versus Secondary Closure
After incision and drainage, the abscess cavity has traditionally been left to heal spontaneously (secondary closure) in order to prevent premature reapproximation of wound edges and abscess recurrence. However, studies — mostly of anogenital abscesses drained in the operating room — have suggested that primary closure after incision and drainage can lead to better outcomes. A systematic review of data from seven randomized trials on 915 patients with cutaneous abscesses, nearly half in the anogenital area (455 assigned to primary closure and 460 assigned to secondary closure), showed that healing time was significantly shorter after primary closure than after secondary closure (7.8 vs. 15.0 days), and recurrence rates were similar.28 Only one study of which we are aware has investigated primary closure of drained cutaneous abscesses in the emergency department. A randomized trial compared primary and secondary closure in 56 adults with abscesses, predominantly on the extremities and caused by MRSA; the 7-day healing and recurrence rates were similar in the two groups (healing rates, 70% and 59%, respectively; recurrence rates, 30% and 29%).22 Primary closure of drained abscesses (Figure 2) should be considered for large incisions (i.e., >2 cm), especially over cosmetically important areas, and may warrant referral to a specialist. Primary closure should not be performed in patients with infected sebaceous cysts or lymph nodes or other infections of chronic skin lesions, patients in whom the adequacy of drainage is in doubt, and patients who have systemic infection or a risk factor for systemic infection (e.g., diabetes).
Antibiotic Treatment
Among 527 patients with a nonperirectal cutaneous abscess who presented in 2008 to U.S. emergency departments that were part of an emerging-infections surveillance network, the abscess was due to community-associated MRSA in 63% of the patients, methicillin-susceptible S. aureus in 15%, beta-hemolytic streptococci in 2%, and other bacteria in the remaining 20%. 29 Almost all MRSA isolates were USA300 strains that have been associated with community-associated infection. Investigations of the efficacy of adjunctive antibiotic treatment for patients with drained cutaneous abscesses have not shown a clear benefit.30 Cure rates with drainage alone are about 85% or higher,31-33 and large studies are therefore required to show relatively small differences in response rates. In the era before community-associated MRSA, investigations consisted of case-series, case–control, and small randomized studies.30 Only recently have adequately powered, randomized, placebo-controlled trials evaluated trimethoprim–sulfamethoxazole (TMP-SMX), which is active against community-associated MRSA, for patients with cutaneous abscesses presenting to emergency departments. One study, which involved 161 children and was powered to detect an absolute between-group difference in clinical response rates of 7 percentage points, showed no significant difference in clinical response rates at 7 days (96% with TMP-SMX and 95% with placebo).32 The other study, which involved 212 adults and was powered to detect a difference in failure rates of 15 percentage points, showed no significant difference in failure rates at 10 days (17% with TMP-SMX and 26% with placebo).33 In each study, new lesion development was less frequent in the group treated with TMP-SMX: in the first study, new lesions developed within 10 days in 13% of children in the TMP-SMX group and in 26% of those in the placebo group; in the second study, new lesions developed within 30 days in 9% and 28% of patients, respectively. These results suggest that antibiotic treatment may have a role in preventing recurrent infection, which is a particular problem for some patients with a high risk of recurrent infection. However, the validity of this observation is unclear because the studies had limitations such as the following: recurrent infection was a secondary outcome, patient dropout was substantial, and there was an imbalance between the study groups at baseline with respect to the presence or absence of a history of abscess. The short-term failure rates and frequency of new lesion development highlight the need for patient education and follow-up.
The Infectious Diseases Society of America (IDSA) recommends systemic antibiotic treatment, in addition to incision and drainage, for patients with severe or extensive disease (e.g., multiple sites of infection) or with rapid disease progression and associated cellulitis, signs and symptoms of systemic illness, associated coexisting conditions or immunosuppression, very young age or advanced age, an abscess in an area difficult to drain (e.g., face, hands, or genitalia), associated septic phlebitis, or an abscess that does not respond to incision and drainage alone.20 In a study involving children with an abscess caused by MRSA, an abscess diameter greater than 5 cm was found to be associated with subsequent hospitalization, although most children were treated with antibiotics that were not active against MRSA, and drainage procedures were not standardized.34 In the trial involving adults, discussed above,33 an abscess diameter greater than 5 cm was not associated with an increased rate of treatment failure.35 Notions that patients with larger lesions, surrounding cellulitis, fever, or coexisting conditions particularly benefit from treatment with adjunctive antibiotics have not been systematically investigated or explored within large randomized trials and therefore are speculative. Two large randomized trials sponsored by the National Institutes of Health (ClinicalTrials.gov numbers, NCT00729937 and NCT00730028) are ongoing and may supply more definitive answers.
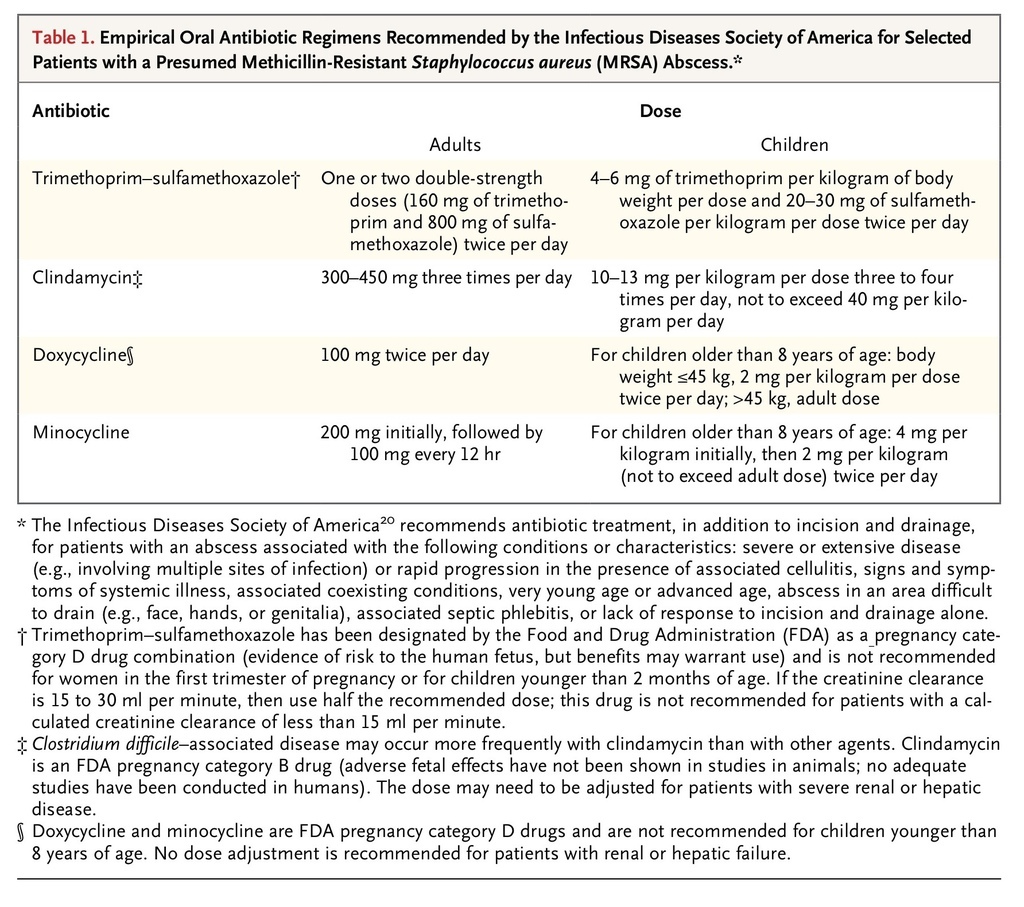
Empirical antibiotic therapy, if prescribed, should have in vitro activity against community-associated MRSA. Most patients who have a minor abscess can be treated as outpatients with inexpensive oral antibiotics. TMP-SMX, clindamycin, and tetracycline have been shown to have in vitro activity against 94% to nearly 100% of more than 300 MRSA isolates tested in a 2008 U.S. emergency department–based surveillance study.29 IDSA-recommended doses of these antibiotics (with doxycycline and minocycline as the preferred tetracycline agents) are provided in Table 1
TABLE 1
Empirical Oral Antibiotic Regimens Recommended by the Infectious Diseases Society of America for Selected Patients with a Presumed Methicillin-Resistant Staphylococcus aureus (MRSA) Abscess.
. Other antibiotics with anti-MRSA activity that have been approved by the Food and Drug Administration for the treatment of skin and soft-tissue infection include vancomycin, linezolid, daptomycin, telavancin, tigecycline, and ceftaroline. 20 The emergence of resistance to clindamycin and tetracyclines has been observed in some communities, and clinicians should therefore be aware of local susceptibility patterns.36-38 The use of tetracyclines should be limited to patients older than 8 years of age.
Although abscess drainage appears to be unlikely to cause bacteremia,39 American Heart Association guidelines indicate that preprocedure use of an antibiotic regimen for treatment of the infection may be reasonable in the case of patients who have the same high-risk cardiac lesions for which antibiotic prophylaxis is recommended in patients undergoing dental procedures.40
Some nonfluctuant lesions without a fluid collection that is detectable by ultrasonography or physical examination may be abscesses at an early stage of development; these lesions often have a central area of induration that may later evolve into a discrete abscess. These lesions are difficult to discriminate from cellulitis, an infection for which the cause is less certain because of the absence of material that can be cultured but that is often thought to be due to streptococci, such as Streptococcus pyogenes. Although TMP-SMX, doxycycline, and minocycline are among the preferred antibiotics for the treatment of community-associated MRSA infections, their activity against streptococci is less well defined than that of beta-lactam agents and clindamycin.20 Therefore, for cases in which early abscess cannot be distinguished from cellulitis, we recommend empirical regimens with activity against both community-associated MRSA and streptococci, such as clindamycin alone or TMP-SMX and a beta-lactam, such as cephalexin or penicillin; follow-up is also recommended, because some abscesses will subsequently require drainage. This approach is consistent with IDSA guidelines for the treatment of these types of skin and soft-tissue infections, although there are no data from clinical trials to support or refute its effectiveness. The likelihood that a given case of cellulitis represents a developing abscess or that it is caused by MRSA is unclear. A recent trial involving 146 patients with uncomplicated cellulitis who were treated with TMP-SMX plus cephalexin or cephalexin alone showed no significant difference in clinical cure rates after 12 days (85% and 82%, respectively; difference, 3 percentage points; 95% confidence interval, −9.3 to 15; P=0.66).41 However, the trial was relatively small — powered to detect only a 13% difference in cure rates — and the 95% confidence interval for the difference in these rates could not rule out the superiority of the former regimen. This same comparison of antibiotics for cellulitis is being investigated in one of the large NIH-sponsored studies mentioned above (NCT00729937), which involves approximately 500 participants.
Wound cultures have helped to define the bacteriologic causes and antimicrobial susceptibility patterns of skin abscesses. However, because the microbiologic causes of nonperirectal cutaneous abscesses are at present relatively predictable, culture results infrequently alter management. Wound cultures have been recommended for patients treated with antibiotics, patients with severe local infection and signs of systemic illness, and patients without an adequate response to initial treatment; cultures have also been recommended if there is concern about a cluster or outbreak.20
Prevention
MRSA colonization is presumed to precede infection. However, although MRSA is now a frequent cause of skin and soft-tissue infection, the point prevalence of colonization in the general population is low. Of 9004 persons in the United States who were screened with the use of nasal cultures in 2004, only 1.5% were found to be colonized with MRSA, with community-associated strains accounting for only 19.7% of the MRSA isolates; 28.6% of the screened persons were colonized with methicillin-susceptible S. aureus.42 These findings suggest that community-associated MRSA may colonize other body sites, may have virulence characteristics that enhance its efficiency in causing infection after colonization as compared with methicillin-susceptible S. aureus, or both. In a study involving 144 children with MRSA skin and soft-tissue infection, 87% were colonized with MRSA, at about equal frequency in the nares and inguinal area, and 27.3% of these MRSA-colonized children's household members were colonized with MRSA.43
Decolonization of the index patient and of household contacts may be considered for patients with recurrent infections,20 and two recent trials have suggested strategies that may be effective. In one randomized trial, involving 183 children with at least one episode of a community-associated S. aureus skin abscess and colonization of the anterior nares, axillae, or inguinal folds, decolonization of the index patient alone was compared with additional decolonization of all household members; decolonization was performed with the use of a 5-day regimen of hygiene, nasal mupirocin treatment, and chlorhexidine body washes.44 Among the 126 patients completing the 12-month follow-up visit, self-reported recurrent skin and soft-tissue infection occurred in 72% in the index group and 52% in the household group (P=0.02); there were no significant between-group differences in colonization eradication (54% and 66%, respectively). This regimen has been recommended for patients with recurrent infections who are found to be colonized with MRSA at any site when the anterior nares and axillary and inguinal fold areas are sampled and who, along with their household members, are highly motivated to attempt this rigorous approach.45
Another approach, directed only at the index patient, was recently described in a prospective trial involving 31 adults with 2 or more confirmed MRSA skin infections during the preceding 6 months.46 Patients were treated with a 10-day regimen of nasal mupirocin twice daily, 3% hexachlorophene body wash daily, and an oral anti-MRSA antibiotic (TMP-SMX, doxycycline, or minocycline) and were followed for another 6 months. This regimen was associated with a reduction in the mean rate of MRSA infection, from 0.84 to 0.03 infections per month; these results suggest that a longer treatment duration and treatment with a systemic antibiotic may increase the effectiveness of the regimen. These investigations lacked a control group, so further studies are needed. If chlorhexidine is used, care should be taken to avoid the eyes and ears, and it should be rinsed off after being applied to the skin. Diluted-bleach baths are a less expensive alternative. Prevention strategies for patients with recurrent infections and household members are described in Table 2
TABLE 2
Prevention Strategies for Patients with Recurrent MRSA Skin and Soft-Tissue Infections and for Their Household Members.
.
SUMMARY
Abscesses are a common form of skin and soft-tissue infection and are increasing in incidence. Although the diagnosis of an abscess can be straightforward, ultrasonography may be helpful in cases in which the abscess is deep, complex, or obscured by extensive cellulitis. A standard approach to incision and drainage remains the mainstay of abscess management, whereas routine packing may be unnecessary. The use of smaller incisions with loop drains and the use of primary closure may be considered in appropriate cases. Adjunctive antibiotic treatment and wound cultures should be limited to patients with severe cases, immunocompromised patients, and those in whom initial therapy is failing. Because of the relatively high failure rates even with optimal treatment, patient education and follow-up are recommended.






 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 