Human papillomavirus (HPV) infection is a common sexually transmitted disease.1-4 Although most infections are benign, persistent infection (repeated detection of an oncogenic type of HPV) is associated with the development of cervical and other anogenital cancers.5-9 Of the more than 30 types of HPV known to infect human genitalia, HPV type 16 (HPV-16) is most commonly linked with cancer, since it is present in 50 percent of cervical cancers and high-grade cervical intraepithelial neoplasias4,10,11 and in 25 percent of low-grade cervical intraepithelial neoplasias.11,12 A vaccine that prevents persistent HPV-16 infection could substantially reduce the incidence of cervical cancer.
The immunogenicity of papillomaviruses involves presentation to the immune system of conformational epitopes displayed on viral capsids composed of L1 protein. Empty viral capsids, termed “virus-like particles,” are synthesized with the use of microbial or cellular expression systems.13-16 Vaccination with L1 virus-like particles derived from species-specific papillomaviruses neutralizes virus17,18 and, in animal models, protects against the development of lesions.17,19,20
In early studies, the HPV-16 L1 virus-like–particle vaccines were generally well tolerated and generated high levels of antibodies against HPV-16.21-23 We conducted a double-blind, multicenter, randomized clinical trial to determine whether such a vaccine could prevent HPV-16 infection in women.
METHODS
Study Population
Between October 1998 and November 1999, 2392 women from 16 centers in the United States were recruited through advertisements on college campuses and in the surrounding communities. Young women, defined as female subjects 16 to 23 years of age, who were not pregnant, reported no prior abnormal Papanicolaou smears, and reported that they had had no more than five male sex partners during their lifetime were eligible for participation. Virgins were enrolled if they were seeking contraception. At enrollment, the women provided written informed consent. The institutional review board at each center approved the protocol. Compensation for subjects was determined independently at each center; amounts ranged from $20 to $225 per visit.
Study Vaccine
The HPV-16 L1 virus-like–particle vaccine (Merck Research Laboratories) consists of highly purified virus-like particles of the L1 capsid of HPV-16. The HPV-16 L1 polypeptide is expressed in yeast (Saccharomyces cerevisiae). Virus-like particles are isolated with the use of standard techniques to achieve a purity of more than 97 percent and adsorbed onto amorphous aluminum hydroxyphosphate sulfate adjuvant without preservative. The HPV-16 vaccine used in this study contained 40 μg of HPV-16 L1 virus-like particles formulated on 225 μg of aluminum adjuvant in a total carrier volume of 0.5 ml. The placebo contained 225 μg of aluminum adjuvant in a total carrier volume of 0.5 ml. Vaccine and placebo were visually indistinguishable.
Women underwent randomization according to a permuted-block design. They were randomly assigned in a 1:1 ratio within study centers to receive three intramuscular injections of either HPV-16 vaccine or placebo at day 0, month 2, and month 6. Body temperature was recorded for five days after each injection. The women recorded all adverse events in a diary for 14 days after each vaccination. In addition, at months 2, 6, and 7, the women were asked by their study clinician about possible adverse events. Adverse events were defined as any sign or symptom of illness or abnormal laboratory test that occurred during the protocol-specified follow-up period and that was not present at enrollment or, if present, had worsened. All such events were called adverse regardless of whether the investigators (who were unaware of the women's treatment assignments) judged them to be related to the vaccine. All adverse events judged by the investigators to be possibly, probably, or definitely related to the vaccine were assumed to be vaccine-related.
Follow-up
At enrollment, the women underwent a gynecologic examination that included the collection of cervical samples for thin-layer Papanicolaou testing (ThinPrep, Cytyc) and cervical swabs, external genital swabs, and cervicovaginal-lavage specimens for HPV-16 DNA testing. Serum was obtained for the measurement of HPV-16 antibody. Follow-up visits were scheduled one month after the third vaccination (month 7), six months after the third vaccination (month 12), and every six months thereafter until month 48. During these visits, specimens were collected for Papanicolaou tests, HPV-16 DNA testing, and measurement of HPV-16 antibodies. The results of HPV-16 tests were not used for clinical care.
Women with high-grade squamous intraepithelial lesions or repeated Papanicolaou tests showing low-grade squamous intraepithelial lesions or atypical squamous cells of undetermined significance were referred for colposcopy. Women with a single Papanicolaou test showing atypical squamous or glandular cells of unknown significance or low-grade squamous intraepithelial lesions were referred according to the local standard of care.
The women with colposcopic abnormalities underwent biopsy. For each abnormal area, two adjacent biopsy specimens were obtained. The first specimen was sent for pathological diagnosis. The second specimen was placed in Specimen Transport Medium (Digene) and submitted for HPV typing with use of the polymerase chain reaction (PCR).
Cytologic and Histologic Analyses
Cervical samples for Papanicolaou testing were deposited in PreservCyt (Cytyc). Thin-layer slides were prepared according to the manufacturer's specifications, screened by cytotechnologists, and reviewed by pathologists at designated cytology laboratories. The results were classified as unsatisfactory if more than 60 percent of the target area of the slide had no epithelial cells. Cellular changes were classified according to the Bethesda system.24
Cervical-biopsy specimens were fixed in 10 percent formalin and embedded in paraffin. Slides were stained with hematoxylin and eosin and reviewed first by a central-laboratory pathologist for purposes of clinical care, and second by an independent panel of four pathologists who had no knowledge of the women's other clinical or laboratory data. Diagnoses were assigned according to the Bethesda and cervical intraepithelial neoplasia systems.24
Detection of HPV DNA
Genital specimens were prepared for PCR according to standard methods. DNA was amplified with the use of HPV type-specific primers based on HPV-16 L1, E6, and E7 genes. PCR products were identified by hybridization with the use of HPV type- and gene-specific oligonucleotides. A positive result was defined as any signal that exceeded the background level associated with an HPV-negative sample of human DNA. Appropriate negative and positive controls were included in each assay. Any sample that tested positive for at least two genes was considered positive. Any sample that tested positive for only one gene was considered positive if, on retesting, it was positive for two or three genes or the same single gene. Validation studies showed that this assay had a probability of more than 95 percent of detecting at least 13 copies per sample. Clinical validation of the assay showed that the 95 percent upper confidence bounds for false negativity and false positivity were 0.7 percent and 0.8 percent, respectively.
HPV-16 Serologic Assay
A competitive radioimmunoassay developed by Merck Research Laboratories was used to quantitate serum HPV-16 antibodies.25 Results were read from a standard curve, corrected for dilution, and reported in arbitrary units (milli-Merck Units, or mMU per milliliter). A fixed cutoff of 5.9 mMU per milliliter (derived by repeatedly testing a panel of positive and negative samples against the standard curve) was used to determine the HPV-16 serologic status of the women. At enrollment, serum from all the women was also evaluated with use of an HPV-16 enzyme-linked immunosorbent assay.26
Primary Case Definition
The primary efficacy hypothesis stated that, as compared with placebo vaccine, HPV-16 L1 virus-like–particle vaccine reduces the incidence of persistent HPV-16 infection. For the primary analysis, a woman met the case definition of persistent HPV-16 infection if she was negative for HPV-16 infection on day 0 and at month 7 and subsequently had HPV-16 DNA detected on two or more consecutive visits four or more months apart; a cervical biopsy showing cervical intraepithelial neoplasia or cervical cancer, as determined by the pathology panel, and HPV-16 DNA in the biopsy tissue and in a swab or lavage sample collected at the antecedent or subsequent visit; or HPV-16 DNA detected only in a sample collected during the last visit before being lost to follow-up. Of 41 women included in the primary efficacy analysis, 31 met only the first criterion, 2 met only the second criterion, 7 met the first and the second criteria, and 1 met only the third criterion. Analyses of safety end points included all adverse events that occurred within 14 days after vaccination and episodes in which body temperature was at least 37.7°C (100°F) within 5 days after vaccination.
Statistical Analysis
The study employed a fixed-number-of-events design. At least 31 cases of persistent HPV-16 infection were required for the study to show a statistically significant reduction in the primary end point (assuming that the true vaccine efficacy was at least 75 percent with a power of at least 90 percent). Accounting for dropouts and women who were HPV-16–positive at enrollment and assuming an event rate of approximately 2 percent per year, we estimated that approximately 2350 women had to be enrolled to yield at least 31 cases of HPV-16 infection. Although the study will continue until all women complete four years of follow-up, the primary analysis was initiated on August 31, 2001, as soon as at least 31 cases were known to have occurred. Thus, the primary analysis includes all safety and efficacy data from visits that occurred on or before that date. Critical data-base fields were audited, and protocol violators were identified before the analysis.
The cohort for the primary analysis of efficacy included women who received the full, correct regimen of study vaccine or placebo and who were HPV-16 seronegative and HPV-16–DNA negative at enrollment and HPV-16–DNA negative at month 7 and who had only negative results on any biopsies performed between enrollment and month 7. As specified in the protocol, women who engaged in sexual intercourse within 48 hours before enrollment or the month 7 visit; who received nonstudy vaccine within the specified time limits relative to vaccination; who received oral or parenteral immunosuppressive agents, immune globulin, or other potentially immunosuppressive blood products; who were enrolled in another study of an investigational agent; or who had a month 7 visit outside the range considered acceptable for determining the month 7 HPV-16 status (14 to 72 days after the third vaccination) were excluded from this analysis.
We conducted a second analysis of the primary end point that included 60 of the 101 women with a general protocol violation. The remaining 41 of these women were excluded from all analyses because they did not receive the full regimen of study vaccine, they were not HPV-16–negative at month 7, or they met both exclusion criteria. A third analysis assessed the efficacy of vaccine in preventing transient or persistent HPV-16 infection (defined as at least one positive test for HPV-16 DNA after the month 7 visit) among HPV-16–negative women.
For all efficacy analyses, a point estimate of vaccine efficacy and the 95 percent confidence interval were calculated on the basis of the observed case split between vaccine and placebo recipients and the accrued person-time. The statistical criterion for success required that the lower bound of the two-sided 95 percent confidence interval for vaccine efficacy exceed 0 percent. For the primary analysis, this corresponds to a test (two-sided α=0.05) of the null hypothesis that the vaccine efficacy equals 0 percent. An exact conditional procedure, which assumes that the numbers of cases in the vaccine and placebo groups are independent Poisson random variables, was used to evaluate vaccine efficacy.27 The immunogenicity analysis, which was based on the sample of women who met protocol-specified criteria for the intervals between visits, provided point estimates of the geometric mean titer for each group and the associated 95 percent confidence intervals. An interim analysis of the primary end point was conducted to plan future studies. Access to results was restricted to persons who were not involved with the present study. No multiplicity adjustment for the interim analysis was made, because the results had no bearing on the conduct of the present study. Although none of the authors were aware of the individual treatment assignments, all authors had access to the data that were unmasked for the purpose of this analysis. All authors take responsibility for the analysis and had authority over decisions concerning publication.
RESULTS
Characteristics of the Cohort Included in the Primary Analysis
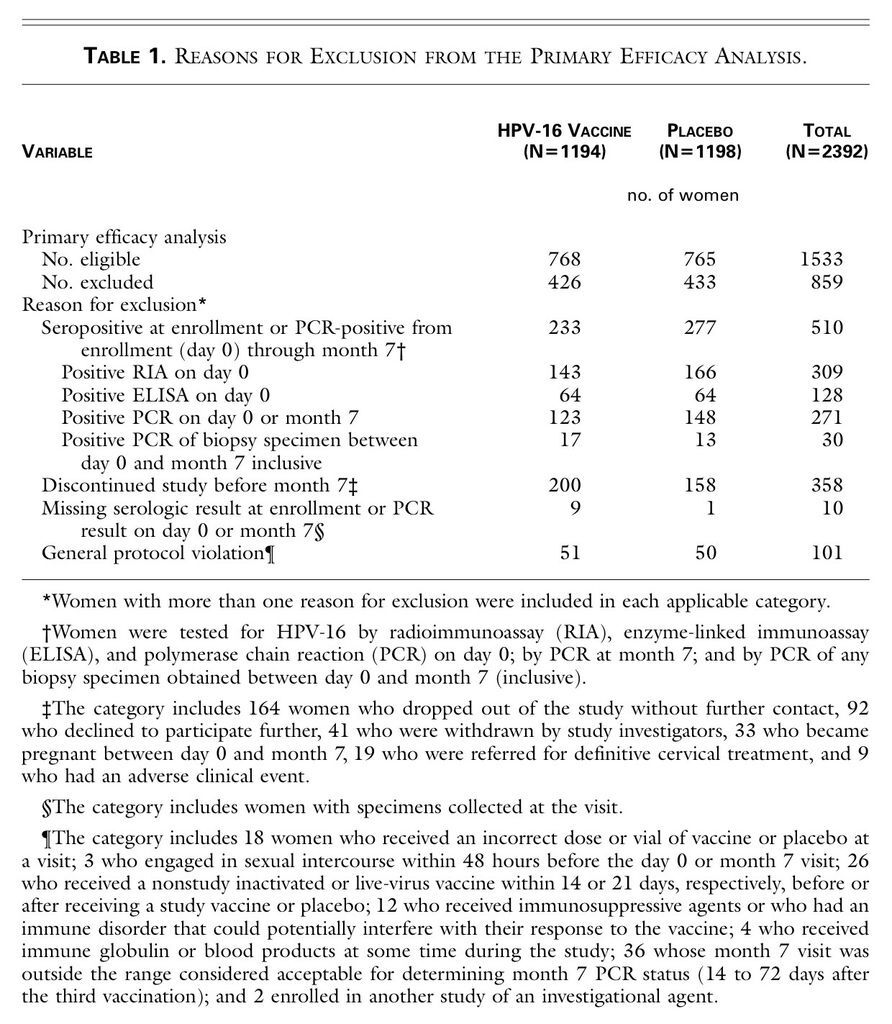
Of the 2392 women enrolled in the study, 1194 received vaccine and 1198 received placebo. Altogether, 1533 women (64 percent of the study cohort) were included in the primary analysis. These women were followed for a median of 17.4 months after completion of the vaccination regimen. The overall proportions of women excluded from this analysis were similar in the two groups. The most common reason for exclusion was evidence of HPV-16 infection at enrollment (Table 1
TABLE 1
Reasons for Exclusion from the Primary Efficacy Analysis.
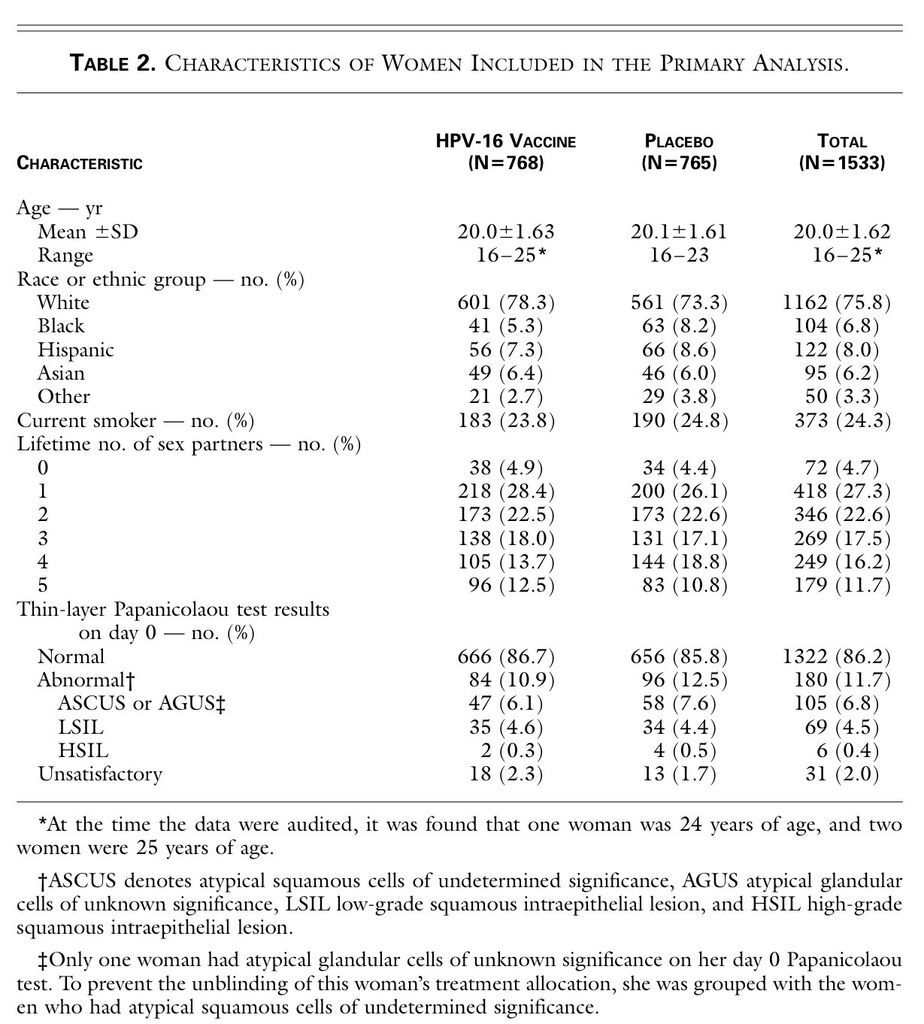
). The median age of the women who were included in the primary analysis was 20 years, and 75.8 percent were white (Table 2
TABLE 2
Characteristics of Women Included in the Primary Analysis.
).
Immunogenicity Analysis
After the third dose (month 7), the geometric mean titer of HPV-16 antibodies was 1510 mMU per milliliter (95 percent confidence interval, 1370 to 1660) among the 619 women who received HPV-16 vaccine and less than 6 mMU per milliliter (all values in the 95 percent confidence interval, <6) among the 631 women who received placebo. For reference, the geometric mean titer of HPV-16 antibodies was 25.7 mMU per milliliter (95 percent confidence interval, 22.4 to 29.4) at enrollment among 337 women who had detectable HPV-16 antibodies on day 0.
Primary Analysis of Persistent HPV-16 Infection
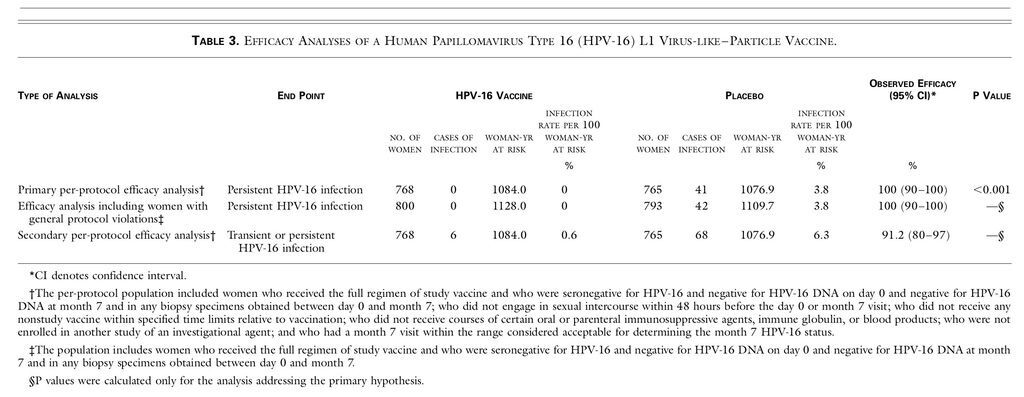
The incidence of persistent HPV-16 infection was 3.8 per 100 woman-years at risk in the placebo group and 0 per 100 woman-years at risk in the vaccine group (100 percent efficacy; 95 percent confidence interval, 90 to 100; P<0.001). Thus, all 41 cases of HPV-16 infection occurred in the placebo group (Table 3
TABLE 3
Efficacy Analyses of a Human Papillomavirus Type 16 (HPV-16) L1 Virus-like–Particle Vaccine.
); 31 were persistent HPV-16 infections without cervical intraepithelial neoplasia, 5 consisted of HPV-16–related cervical intraepithelial neoplasia grade 1, 4 consisted of HPV-16–related cervical intraepithelial neoplasia grade 2, and 1 occurred in a woman who was HPV-16–DNA positive for the first time on the last visit before she was lost to follow-up. An additional 44 cases of cervical intraepithelial neoplasia that were not associated with HPV-16 infection were detected, 22 among placebo recipients and 22 among vaccine recipients.
Secondary Analyses
Including the 60 women with general protocol violations, there were 800 vaccine recipients and 793 placebo recipients in the efficacy analysis. One additional case of persistent HPV-16 infection (without cervical intraepithelial neoplasia) was detected among placebo recipients (Table 3).
Of the 1533 women included in the primary analysis, 74 were positive for HPV-16 DNA at least once after month 7 (Table 3). None of the 33 women who were transiently positive for HPV-16 DNA at a single visit (6 vaccine recipients and 27 placebo recipients) had HPV-16–related cervical intraepithelial neoplasia.
Tolerability of Vaccine
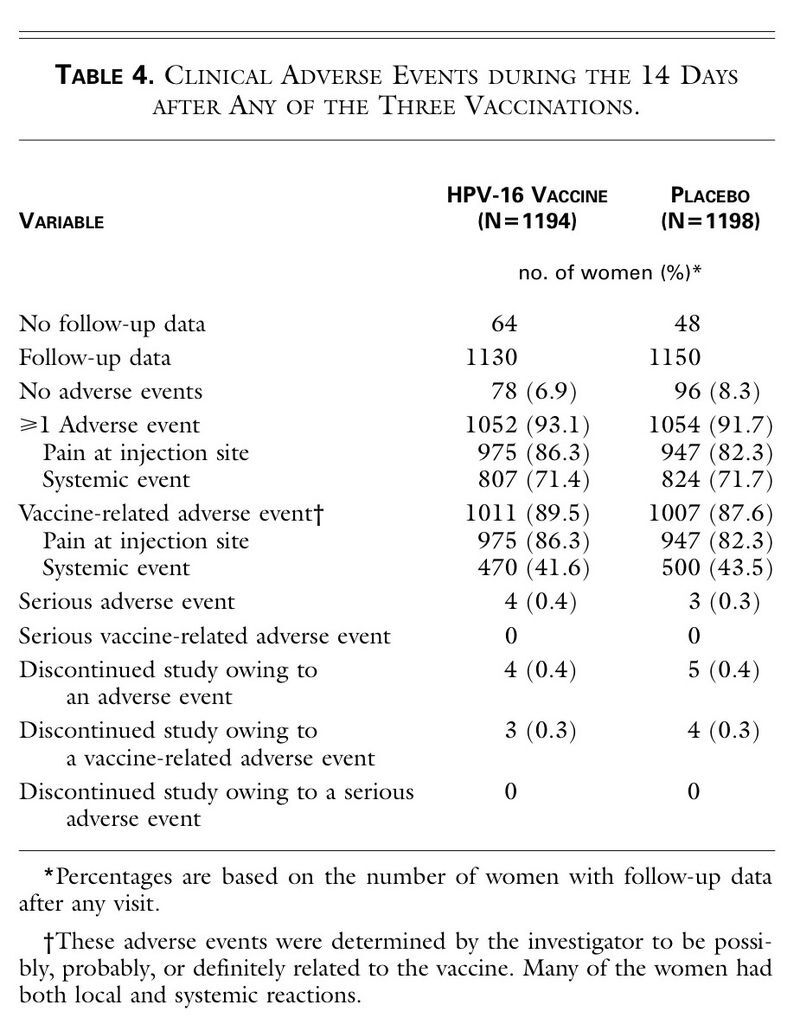
The incidence of adverse events was similar in the two groups (Table 4
TABLE 4
Clinical Adverse Events during the 14 Days after Any of the Three Vaccinations.
). The most frequent adverse experience was pain at the injection site. The percentages of women with temperatures of at least 37.7°C (100°F) were similar in the two groups.
DISCUSSION
Our data provide evidence of a highly efficacious prophylactic vaccine against HPV infection. A three-dose regimen of HPV-16 vaccine reduced the incidence of persistent HPV-16 infection; all 41 cases of new HPV-16 infection, including 9 cases of HPV-16–related cervical intraepithelial neoplasia, occurred among placebo recipients (vaccine efficacy of 100 percent).
The analysis that included women with a general protocol violation — an approach that more closely approximated the use of vaccine under typical conditions — also demonstrated vaccine efficacy of 100 percent. Furthermore, the analysis that included cases of HPV-16 infection defined on the basis of a single positive HPV-16–DNA test showed a high rate of efficacy (91.2 percent). Only 6 cases in which tests were positive at a single visit occurred among vaccine recipients, whereas 27 cases were expected on the basis of the observed rate in the placebo group. Assuming that all women with a single positive test had a new infection (and the results did not involve contamination of the sample or a reactivation of infection), the data support the possibility that sterilizing immunity developed in some women.
Of women who received HPV-16 vaccine, 99.7 percent seroconverted. At month 7, the geometric mean titer of HPV-16 antibodies in these women was 58.7 times as high as the geometric mean titer among women with serologic evidence of natural HPV-16 infection at enrollment. A small immunogenicity study of a baculovirus-derived HPV-16 virus-like–particle vaccine reported similar results.21 The duration of antibodies and protection remains to be determined.
Although the vaccine was generally well tolerated, a slightly higher percentage of women in the vaccine group than in the placebo group did not complete the vaccination series or withdrew shortly thereafter, suggesting that the vaccine may have been associated with reduced tolerability. There were no serious vaccine-related adverse events. The most common adverse event reported by both vaccine recipients and placebo recipients was pain at the injection site. This finding is consistent with those of other studies of inactivated or subunit vaccines.28,29
The percentage of women who were enrolled but who were excluded from the primary efficacy analysis was similar in the two groups, but it was higher than the rates reported in other vaccine studies. The most common reason for exclusion was the detection of HPV-16 DNA or antibodies in samples collected during the visit at day 0 or month 7. This exclusion was made because there are no data showing that vaccines based on papillomavirus-virus-like particles provide postinfection protection against either persistent infection or lesions. Women who were infected with other types of HPV were not excluded from the study, because evidence suggests that cross-protection at this level is either minimal or absent.30-32
The primary reason to immunize against HPV-16 infection is to prevent cervical cancer. This end point would be difficult to study for ethical and scientific reasons. Persistent HPV-16 infection8 is a reasonable surrogate end point, since approximately 50 percent of cervical cancers are associated with HPV-16 infection.10 Moreover, a large body of evidence has shown that HPV-16 is a potent human carcinogen.5 The fact that all nine cases of HPV-16–related cervical intraepithelial neoplasia occurred among the placebo recipients constitutes encouraging evidence of the efficacy of the vaccine, but a larger study is required to prove that clinical disease is prevented by vaccination.
We evaluated a monovalent HPV-16 vaccine. From a public health perspective, a vaccine that prevents infection with a broad spectrum of types of HPV would be more advantageous. Multivalent vaccines that include other common types of HPV are being evaluated.
The administration of HPV-16 L1 virus-like–particle vaccine to young women reduced the incidence of HPV-16 infections and related cervical intraepithelial neoplasia. Immunizing HPV-16–negative women may reduce their risk of cervical cancer.





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 