Arthur Kavanaugh, Philip J Mease, Juan J Gomez-Reino, et al.
Correspondence to Dr Arthur Kavanaugh, Division of Rheumatology, Allergy and Immunology, Department of Medicine, University of California, San Diego 9500 Gilman Drive, Mail Code 0943, La Jolla, CA 92093-0943, USA; akavanaugh@ucsd.edu
Abstract
Objectives Apremilast, an oral phosphodiesterase 4 inhibitor, regulates inflammatory mediators. Psoriatic Arthritis Long-term Assessment of Clinical Efficacy 1 (PALACE 1) compared apremilast with placebo in patients with active psoriatic arthritis despite prior traditional disease-modifying antirheumatic drug (DMARD) and/or biologic therapy.
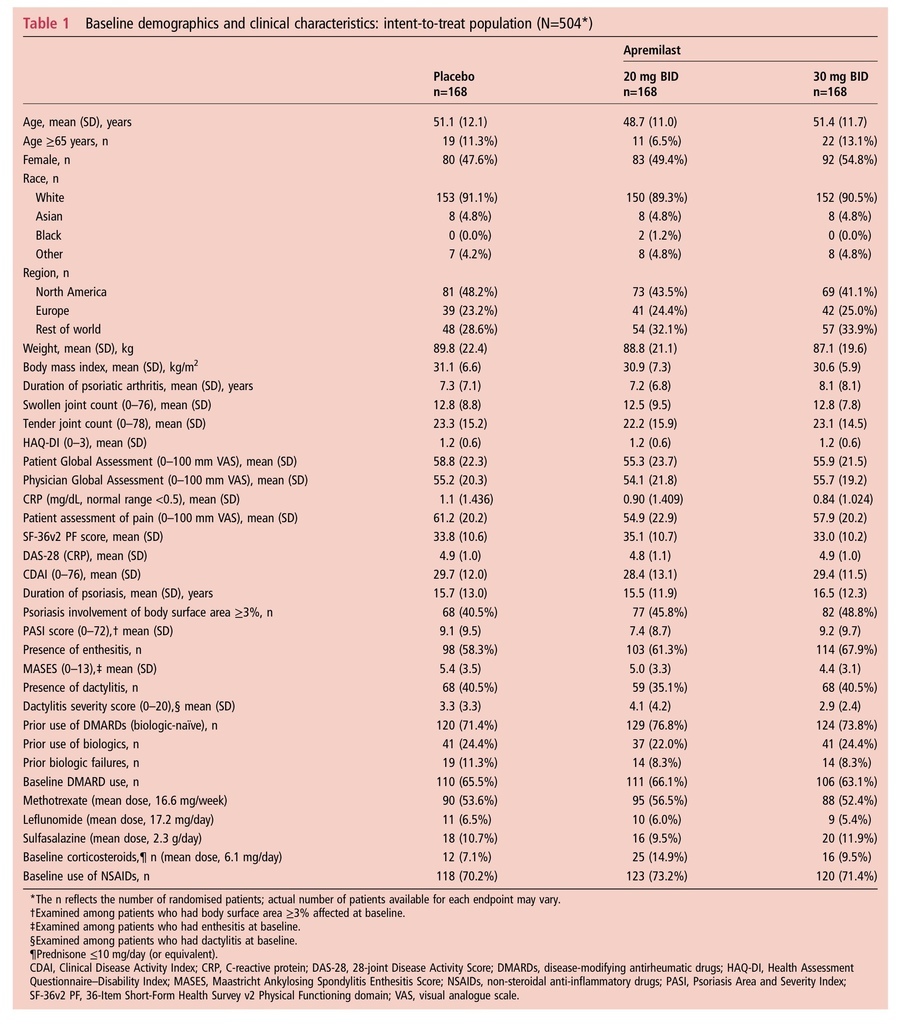
Methods In the 24-week, placebo-controlled phase of PALACE 1, patients (N=504) were randomised (1:1:1) to placebo, apremilast 20 mg twice a day (BID) or apremilast 30 mg BID. At week 16, patients without ≥20% reduction in swollen and tender joint counts were required to be re-randomised equally to either apremilast dose if initially randomised to placebo or remained on their initial apremilast dose. Patients on background concurrent DMARDs continued stable doses (methotrexate, leflunomide and/or sulfasalazine). Primary outcome was the proportion of patients achieving 20% improvement in modified American College of Rheumatology response criteria (ACR20) at week 16.
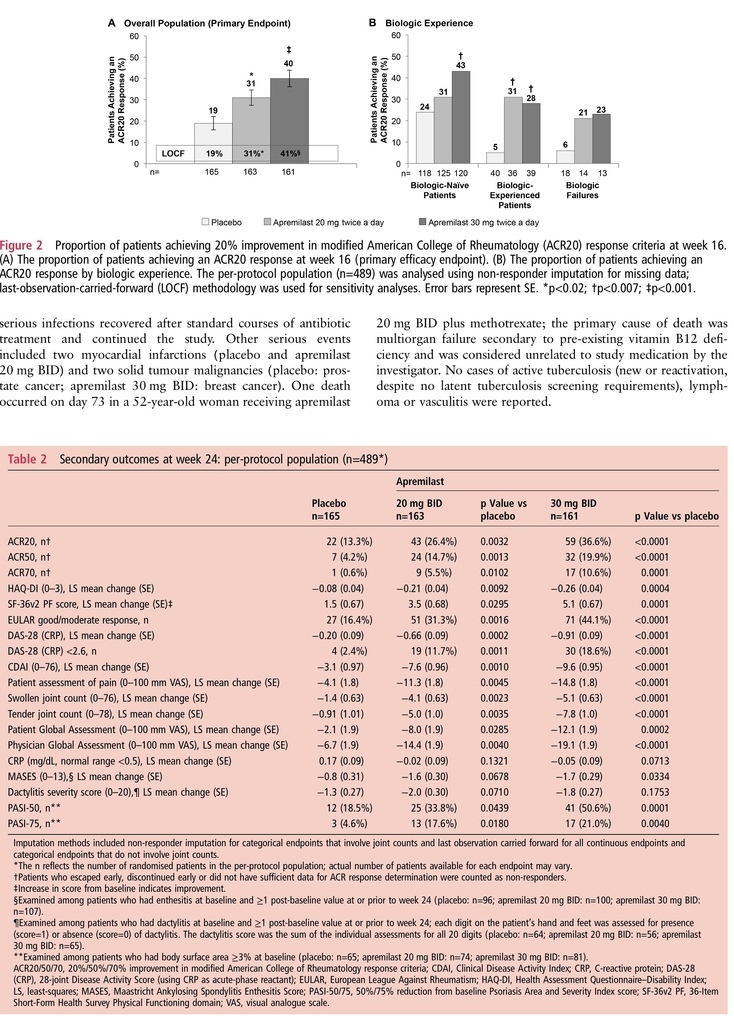
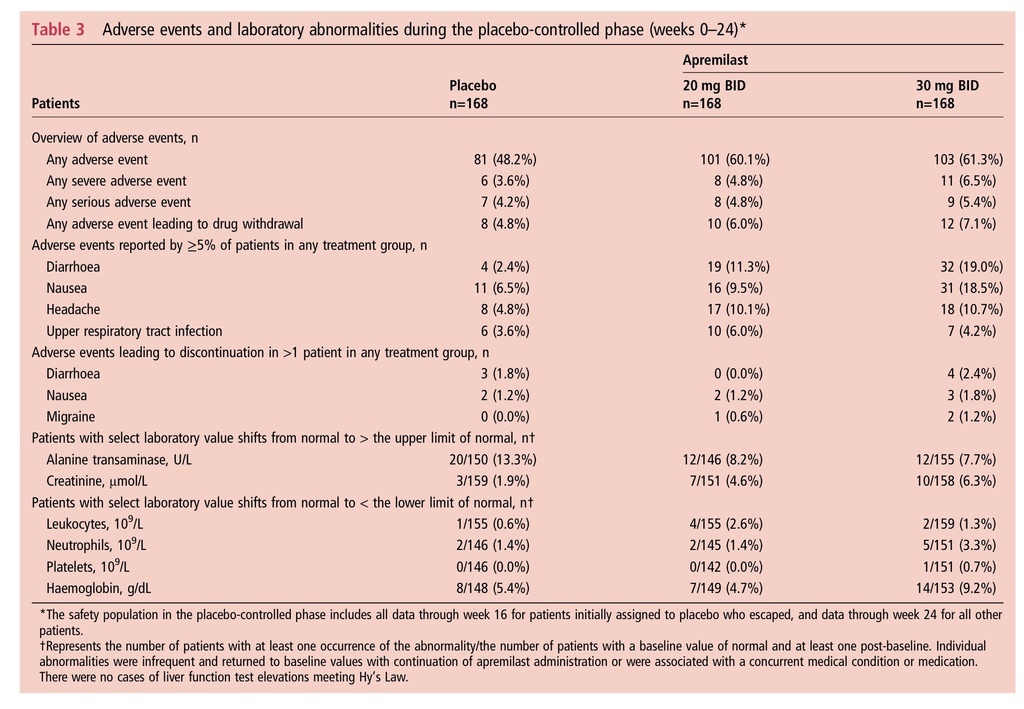
Results At week 16, significantly more apremilast 20 mg BID (31%) and 30 mg BID (40%) patients achieved ACR20 versus placebo (19%) (p<0.001). Significant improvements in key secondary measures (physical function, psoriasis) were evident with both apremilast doses versus placebo. Across outcome measures, the 30-mg group generally had higher and more consistent response rates, although statistical comparison was not conducted. The most common adverse events were gastrointestinal and generally occurred early, were self-limiting and infrequently led to discontinuation. No imbalance in major adverse cardiac events, serious or opportunistic infections, malignancies or laboratory abnormalities was observed.
Conclusions Apremilast was effective in the treatment of psoriatic arthritis, improving signs and symptoms and physical function. Apremilast demonstrated an acceptable safety profile and was generally well tolerated.
Clinical trial registration number NCT01172938.






 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 