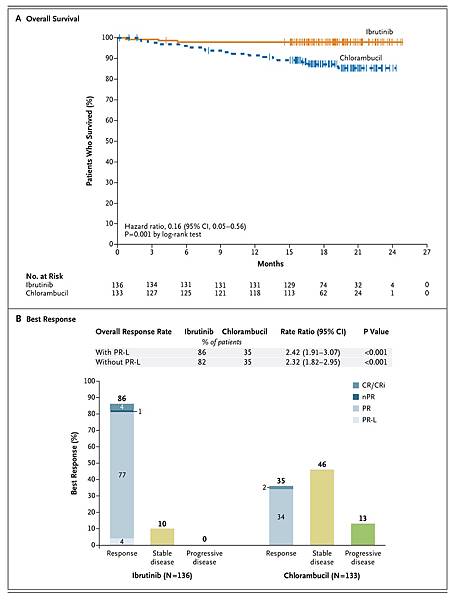
Survival was significantly improved with ibrutinib versus chlorambucil.
Treatment for patients with relapsed and high-risk chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) has been revolutionized by agents targeting the B-cell receptor pathway.
To test the frontline therapeutic activity of the Bruton tyrosine kinase inhibitor ibrutinib, investigators conducted an industry-sponsored, international, open-label, randomized, phase III trial comparing ibrutinib with chlorambucil in 269 previously untreated patients (median age, 73). The study arms were balanced for the adverse prognostic markers del(11q) and unmutated IGHV. Patients with the poor-risk biomarker del(17p) were excluded.
Progression-free survival (the primary endpoint) at 18 months was improved with ibrutinib versus chlorambucil in both IGHV-mutated and unmutated CLL (89% vs. 47%–51%), as were overall survival at 24 months (98% vs. 85%; P=0.001), overall response (86% vs. 35%), and rates of sustained increases in hemoglobin and platelet levels. Treatment was discontinued in fewer ibrutinib recipients than chlorambucil recipients (9% vs. 23%).
COMMENT
Ibrutinib has become a standard of care in relapsed CLL or SLL, and these results now support its use as first-line therapy in addition to its current indication for del(17p) CLL. Important questions remain regarding longer-term efficacy and safety compared with anti-CD20 immunotherapy and chemotherapy as well as the role for ibrutinib combination regimens. Biomarker and minimal residual disease assessment may ultimately be useful to guide the targeted agent or regimen of choice and the duration of treatment.
Michael E. Williams, MD, ScM reviewing Burger JA et al. N Engl J Med 2015 Dec 6.











 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 