N Engl J Med 2018; 379:1509-1518 DOI: 10.1056/NEJMoa1805819
Abstract
BACKGROUND
Aspirin is a well-established therapy for the secondary prevention of cardiovascular events. However, its role in the primary prevention of cardiovascular disease is unclear, especially in older persons, who have an increased risk.
METHODS
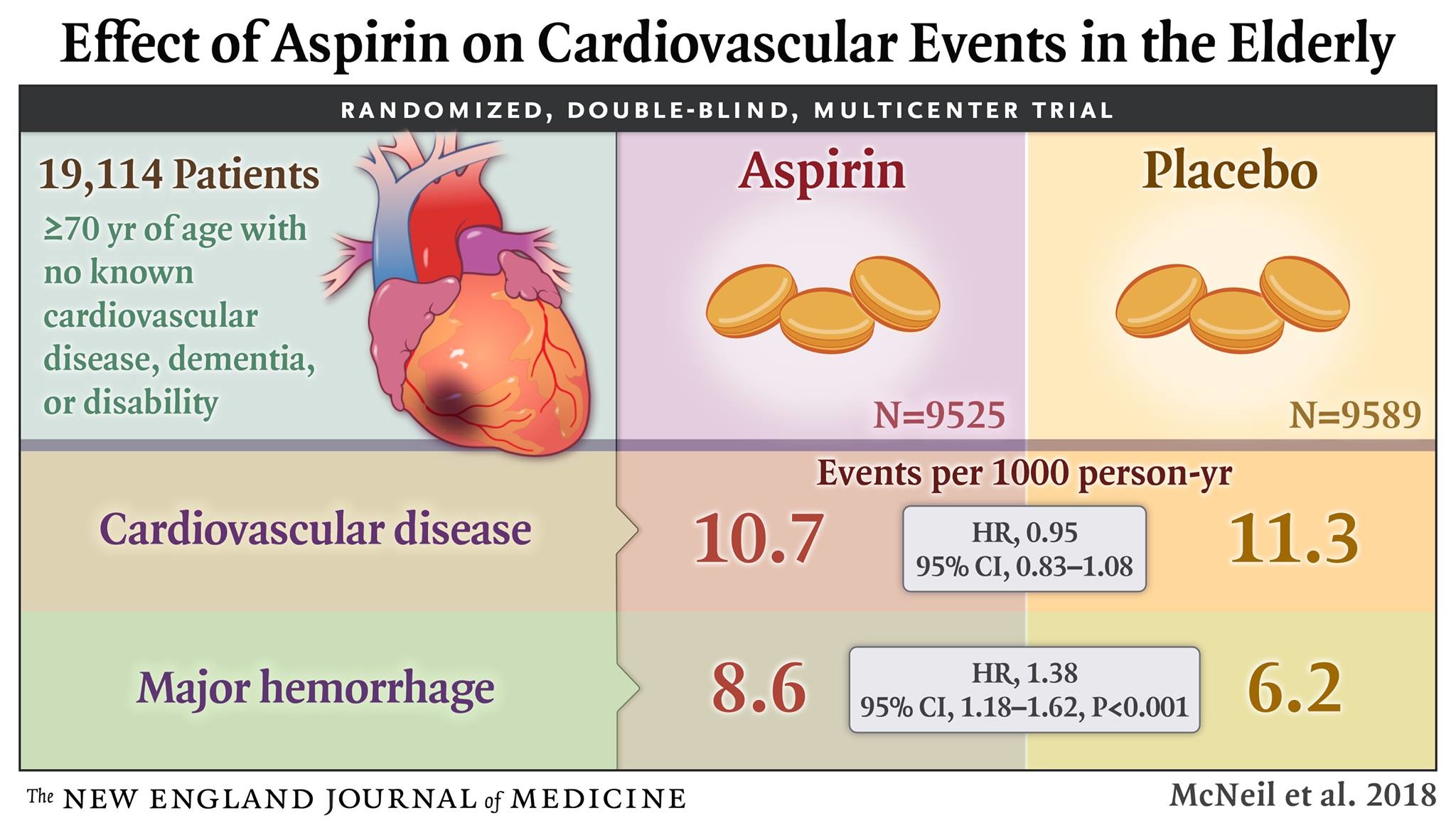
From 2010 through 2014, we enrolled community-dwelling men and women in Australia and the United States who were 70 years of age or older (or ≥65 years of age among blacks and Hispanics in the United States) and did not have cardiovascular disease, dementia, or disability. Participants were randomly assigned to receive 100 mg of enteric-coated aspirin or placebo. The primary end point was a composite of death, dementia, or persistent physical disability; results for this end point are reported in another article in the Journal. Secondary end points included major hemorrhage and cardiovascular disease (defined as fatal coronary heart disease, nonfatal myocardial infarction, fatal or nonfatal stroke, or hospitalization for heart failure).
RESULTS
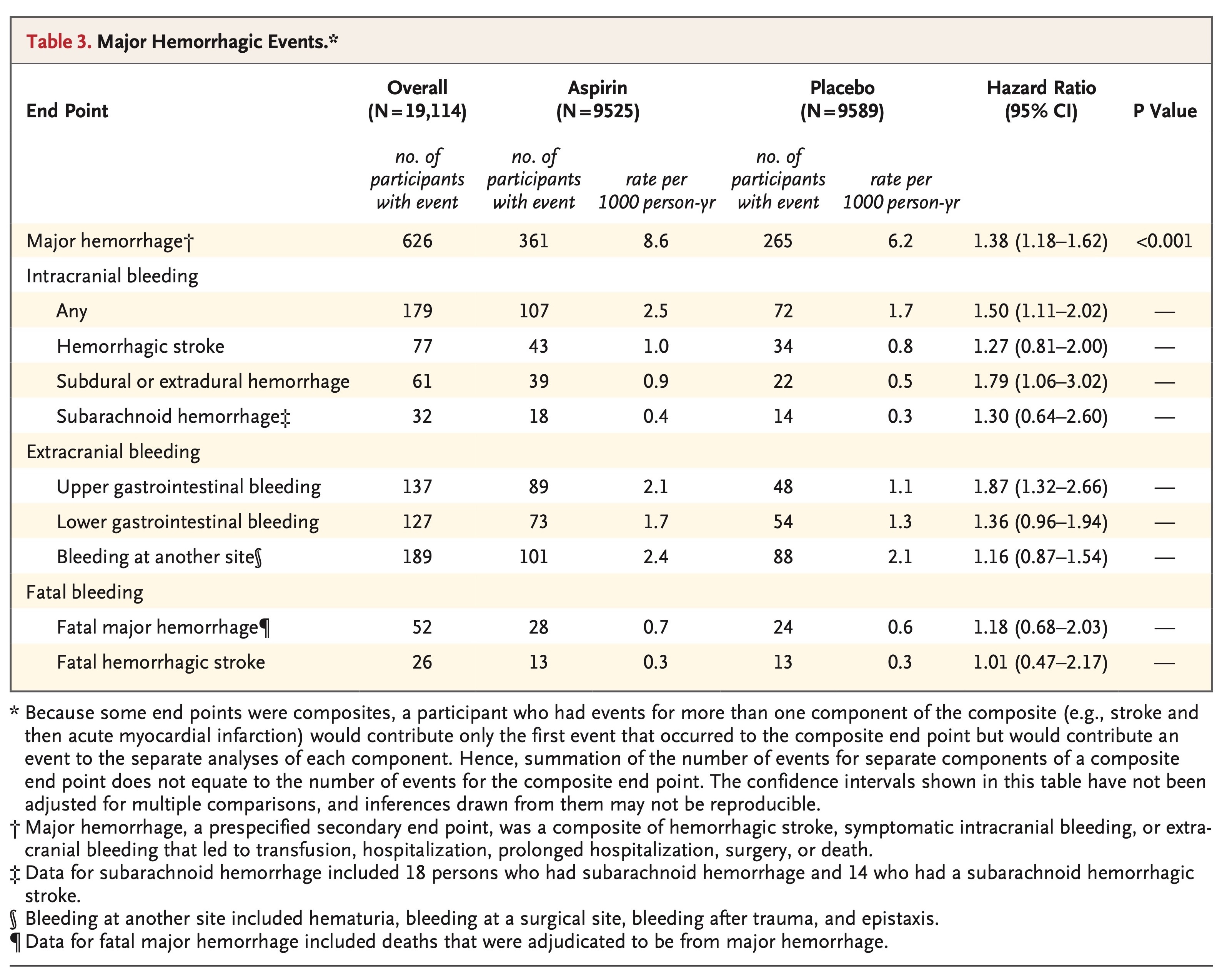
Of the 19,114 persons who were enrolled in the trial, 9525 were assigned to receive aspirin and 9589 to receive placebo. After a median of 4.7 years of follow-up, the rate of cardiovascular disease was 10.7 events per 1000 person-years in the aspirin group and 11.3 events per 1000 person-years in the placebo group (hazard ratio, 0.95; 95% confidence interval [CI], 0.83 to 1.08). The rate of major hemorrhage was 8.6 events per 1000 person-years and 6.2 events per 1000 person-years, respectively (hazard ratio, 1.38; 95% CI, 1.18 to 1.62; P<0.001).
CONCLUSIONS
The use of low-dose aspirin as a primary prevention strategy in older adults resulted in a significantly higher risk of major hemorrhage and did not result in a significantly lower risk of cardiovascular disease than placebo. (Funded by the National Institute on Aging and others; ASPREE ClinicalTrials.gov number, NCT01038583.)
Dramatic increases in life expectancy over the past century have led to a substantial demographic shift toward an aging society in many countries. Thus, maintaining good health in older persons is an increasingly important public health aim. Cardiovascular diseases are among the principal causes of disability and death in older persons, and therefore, preventive interventions for such diseases are a high priority.1-3
Low-dose aspirin is among the most widely used agents for the prevention of cardiovascular disease.4-8 Its efficacy has been established in secondary prevention trials, in which the benefits associated with reducing the rates of both myocardial infarction and ischemic stroke have appeared to outweigh the risk of hemorrhage.9,10 In primary prevention trials, involving participants in whom the risk of cardiovascular disease was typically lower than the risk seen in secondary prevention trials, the risks and benefits of low-dose aspirin have been more finely balanced. The role of low-dose aspirin as a primary prevention strategy is debated.11-13
In elderly populations, the risk of cardiovascular disease is higher and the potential benefits of aspirin may accordingly be greater than in younger populations. However, an increased risk of bleeding has also been observed in the elderly age group.10,14-17 Because a limited number of older persons have been included in previous primary prevention trials, the risk–benefit balance in this age group is unknown.18
In the primary analysis of the Aspirin in Reducing Events in the Elderly (ASPREE) trial, now published in the Journal,19,20 we report that daily use of low-dose aspirin did not prolong disability-free survival among the elderly. Here, we report findings from the ASPREE trial regarding the effect of aspirin on the prespecified secondary end points of cardiovascular disease and major hemorrhage. We also present analyses of the nonprespecified end point of major adverse cardiovascular events.
Methods
TRIAL DESIGN
Details of the trial rationale and design have been reported previously.21-23 The protocol and statistical analysis plan are available with the full text of this article at NEJM.org. In brief, we conducted this randomized, double-blind, placebo-controlled clinical trial to evaluate the effect of daily use of 100 mg of enteric-coated aspirin in community-dwelling older adults.
Bayer Pharma (Germany) provided the trial drug (aspirin) and placebo but had no other role in the trial. The Department of Epidemiology and Preventive Medicine at Monash University in Australia coordinated the data collection and was responsible for statistical analyses. Coordination of the trial and monitoring of sites was conducted by this department in Australia and by the Berman Center for Outcomes and Clinical Research in the United States.
The trial was approved by the ethics committee at each participating center, and all the participants provided written informed consent before enrollment. The trial funder (the National Institute on Aging) and an independent data and safety monitoring board, whose members had been appointed by the National Institute on Aging, reviewed reports on the accumulating data at regular intervals. Trial centers were randomly audited; a data-quality committee monitored for the completeness and accuracy of the data, and the independent data and safety monitoring board monitored for safety. All the authors vouch for the accuracy and completeness of the data and analyses and for the fidelity of the trial to the protocol.
TRIAL PARTICIPANTS
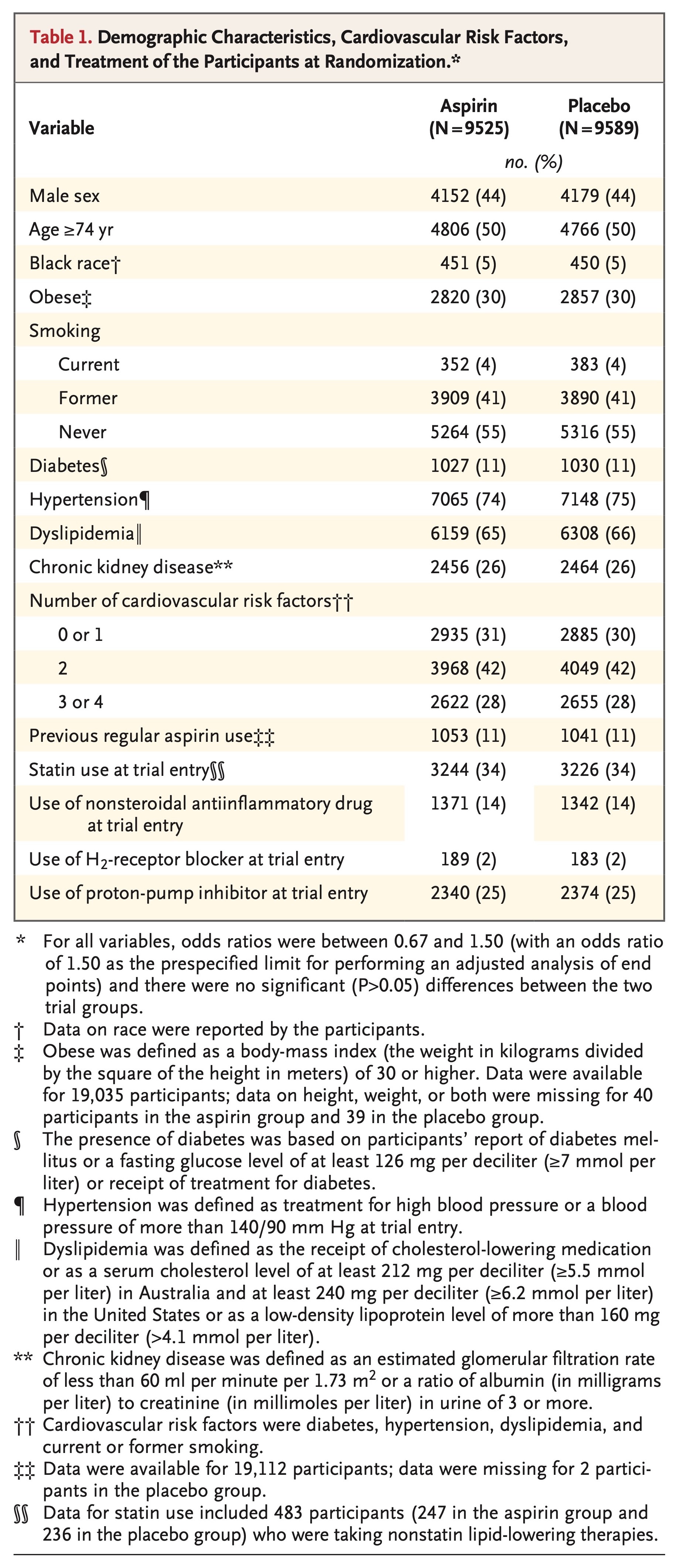
Eligible participants were community-dwelling adults from Australia and the United States who were 70 years of age or older (or ≥65 years of age among blacks and Hispanics in the United States). Eligibility criteria are provided in Table S1 in the Supplementary Appendix, available at NEJM.org. Participants were required to be free from overt coronary heart disease, overt cerebrovascular disease, atrial fibrillation, a clinical diagnosis of dementia, clinically significant physical disability, a high risk of bleeding, anemia, and a known contraindication to or inability to take aspirin. Key exclusion criteria were the current regular use of an anticoagulant or antiplatelet medication other than aspirin, a systolic blood pressure of 180 mm Hg or more or a diastolic blood pressure of 105 mm Hg or more, a medical indication for or contraindication to regular aspirin therapy, or the presence of a condition that, in the opinion of the primary care physician, was likely to result in death within 5 years. Participants were allowed to take other nonsteroidal antiinflammatory drugs (NSAIDs), with the recommendation to take the lowest dose for the shortest duration necessary.
TRIAL PROCEDURES
Participants who had a rate of adherence to pill ingestion, as assessed by pill count, of 80% or greater during a 1-month placebo run-in phase were randomly assigned, in a 1:1 ratio, to receive aspirin or placebo. Randomization was stratified according to trial center and age (65 to 79 years or ≥80 years). Annual in-person visits and medical-record reviews were supplemented by regular telephone calls to encourage retention in the trial and facilitate the collection of clinical data (Table S2 in the Supplementary Appendix).
Trial participants, investigators, and general practitioner associate investigators were unaware of the trial-group assignments until the publication of this article. Adherence to the trial intervention was assessed annually by means of tablet counts on returned bottles of aspirin or placebo.
Committees whose members were unaware of the trial-group assignments were responsible for adjudication of all potential clinical end-point events. Descriptions of the definitions and processes used for adjudication are provided in the Supplementary Appendix. Additional information regarding the adjudication of major hemorrhagic events has been published previously.24 Data on causes of death are provided in a separate article in the Journal.20
CARDIOVASCULAR EVENTS
A prespecified secondary end point of the trial was cardiovascular disease, which was a composite of fatal coronary heart disease, nonfatal myocardial infarction, fatal or nonfatal stroke, or hospitalization for heart failure. The individual components of this end point were not prespecified as separate end points but were evaluated in a post hoc analysis to assist in the interpretation of the composite end point.
Fatal coronary heart disease was defined as death from myocardial infarction, sudden cardiac death, or any other death in which the underlying cause was considered to be coronary heart disease. Fatal stroke was defined as any death in which the underlying cause was an obstruction or rupture in the intracranial or extracranial cerebral arterial system. Fatal cardiovascular disease was defined as any death in which the underlying cause was coronary heart disease or stroke.
The definition of nonfatal myocardial infarction was based on joint guidelines of the European Society of Cardiology and the American College of Cardiology.25 Hospitalization for heart failure was defined as any unplanned stay, overnight or longer, in a hospital environment (emergency department, observation unit, or inpatient-care unit) or a similar facility, for which the principal reason for admission was heart failure. The definition of nonfatal stroke was based on the World Health Organization definition of rapidly developing clinical signs of focal or global disturbance of cerebral function lasting more than 24 hours (unless interrupted by surgery or death), with no apparent cause other than ischemic or hemorrhagic cerebrovascular disease.26 In both Australia and the United States, source documentation — including clinical notes, hospitalization records, and imaging studies (computed tomographic scans or magnetic resonance images) — was requested for events that were thought to be potential end-point events.
The nonprespecified end point of major adverse cardiovascular events was a composite of fatal coronary heart disease (excluding death from heart failure), nonfatal myocardial infarction, or fatal or nonfatal ischemic stroke. This end point included the conditions related to ischemia and thrombosis that are most likely to be affected favorably by low-dose aspirin.
MAJOR HEMORRHAGIC EVENTS
Another prespecified secondary end point was major hemorrhage, which was a composite of hemorrhagic stroke, symptomatic intracranial bleeding, or clinically significant extracranial bleeding. Clinically significant extracranial bleeding was defined as bleeding that led to transfusion, hospitalization, prolongation of hospitalization, surgery, or death.
STATISTICAL ANALYSIS
In intention-to-treat analyses, Cox proportional-hazards models were used to compare the aspirin group with the placebo group with regard to time-to-event end points. Cause-specific hazards were compared between groups, with deaths from causes other than those included in the end point of interest treated as censoring events. Confidence intervals were not adjusted for multiple comparisons, and P values are not presented for secondary or nonprespecified end points, except for major hemorrhage as a safety end point. Proportional-hazards assumptions were tested as a null hypothesis of a zero slope in a regression model of scaled Schoenfeld residuals against time; all P values were found to be greater than 0.1, indicating satisfaction of the assumption for all end points. Cumulative incidences were used to show event risk. The cumulative incidences were based on competing-risks regression models, which were stratified according to trial group and which allowed for the competing risk of death from causes other than those included in the end point of interest.27
Subgroups of potential relevance to the risk of cardiovascular disease that were specified in the statistical analysis plan included sex, age (younger than the median age vs. the median age or older), country of residence (Australia vs. the United States), race or ethnic group (white in Australia, white in the United States, black, Hispanic, or other), smoking (never smoked, former smoker, or current smoker), body-mass index (the weight in kilograms divided by the square of the height in meters; <20 [underweight], 20 to 24 [normal weight], 25 to 29 [overweight], or ≥30 [obese]), previous regular use of aspirin (yes vs. no), and the presence of diabetes, hypertension, and dyslipidemia at baseline (yes vs. no, for each condition).23 Nonprespecified subgroups that were also considered to be of interest included the number of cardiovascular risk factors (diabetes, hypertension, dyslipidemia, and current or former smoking) that were present (0 or 1, 2, or 3 or 4), the presence of chronic kidney disease (yes vs. no), and the use of statins or NSAIDs at randomization (yes vs. no, for each type of drug). A nonprespecified subgroup of potential relevance to the risk of major hemorrhage was the use of proton-pump inhibitors at randomization (yes vs. no). Interaction terms in Cox proportional-hazards models were used to test for heterogeneity of effect between subgroups.
Results
The intervention phase ceased on June 12, 2017. All analyses were restricted to events that occurred through that date. The median follow-up was 4.7 years; 1.5% of the participants in the aspirin group and 1.6% of those in the placebo group had been lost to follow-up by the end of the trial, and 1.2% of the participants in each group had withdrawn consent (Fig. S1 in the Supplementary Appendix). In the final 12 months of the trial, 62% of the participants in the aspirin group and 64% of those in the placebo group were still taking the assigned trial intervention.
CARDIOVASCULAR EVENTS
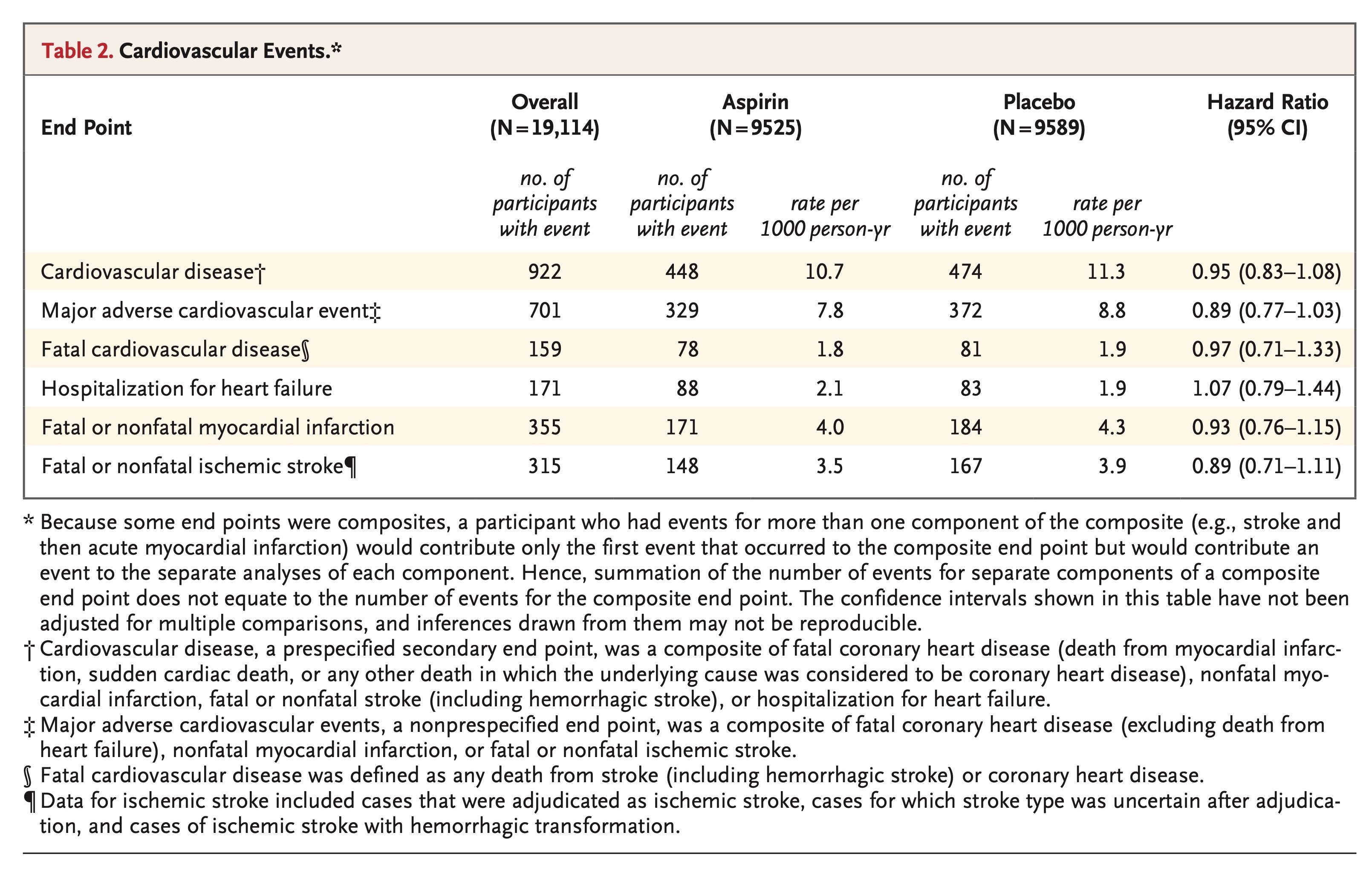
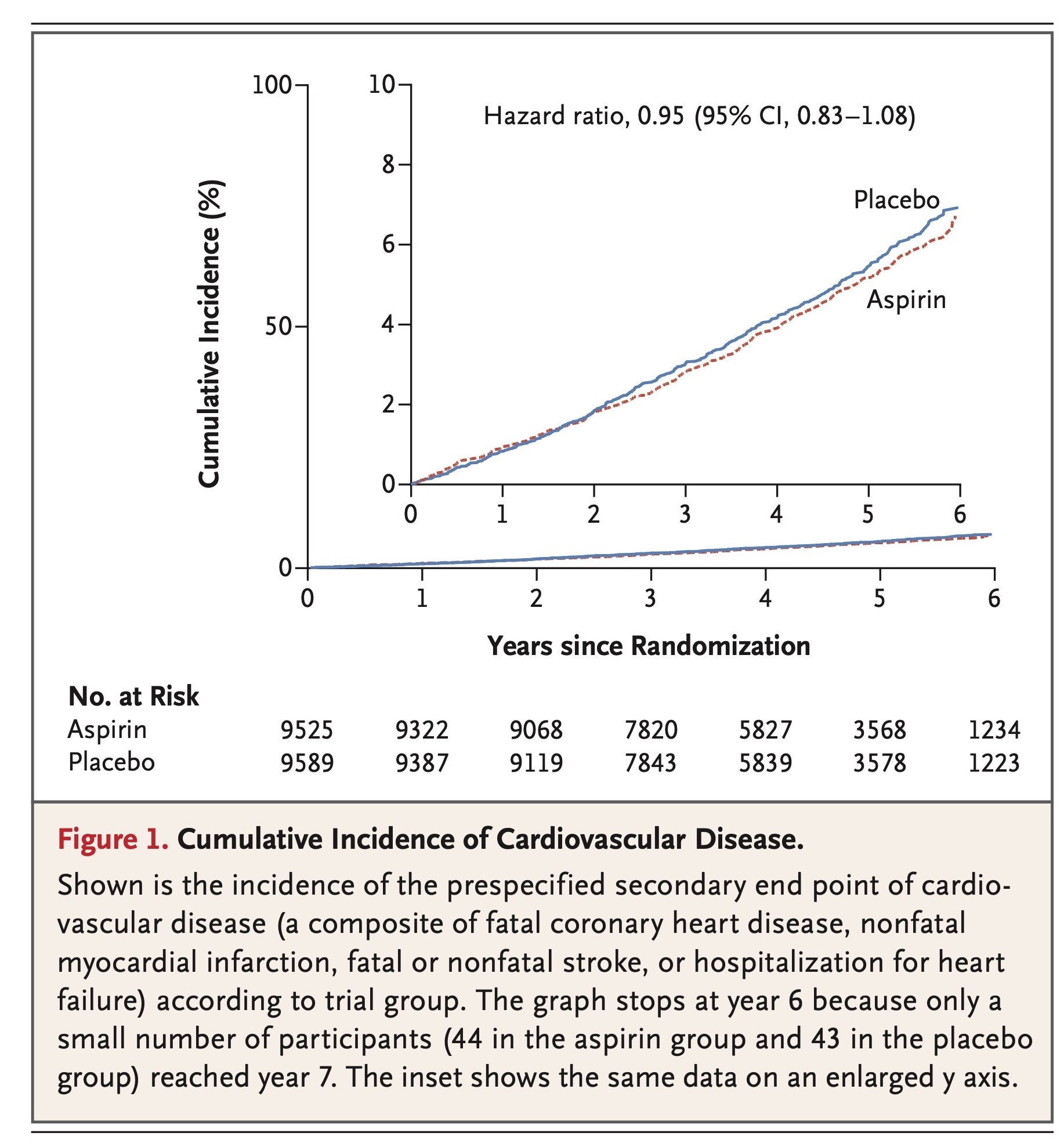
Rates of cardiovascular events are shown in Table 2 and Figure 1, and further details, including data on subclassification of the events, are provided in Table S3 in the Supplementary Appendix. The rate of the prespecified secondary end point of cardiovascular disease did not differ significantly between the aspirin group and the placebo group (10.7 events per 1000 person-years of follow-up and 11.3 events per 1000 person-years, respectively; hazard ratio, 0.95; 95% confidence interval [CI], 0.83 to 1.08).
The rate of major adverse cardiovascular events was 7.8 events per 1000 person-years in the aspirin group and 8.8 events per 1000 person-years in the placebo group (hazard ratio, 0.89; 95% CI, 0.77 to 1.03) (Table 2, and Fig. S2 in the Supplementary Appendix). Individual rates of myocardial infarction, ischemic stroke, fatal cardiovascular disease, and hospitalization for heart failure were similar in the two groups (Table 2, and Figs. S3 and S4 in the Supplementary Appendix).
There was no evidence of a differential effect of aspirin on the risk of cardiovascular disease in any analyses of the prespecified subgroups or in post hoc analyses of subgroups of potential relevance to the risk of cardiovascular disease (Figs. S5 and S6 in the Supplementary Appendix). There was also no evidence of an interaction between trial group and any of the subgroups with regard to the end point of major adverse cardiovascular events (Figs. S7 and S8 in the Supplementary Appendix).
MAJOR HEMORRHAGIC EVENTS
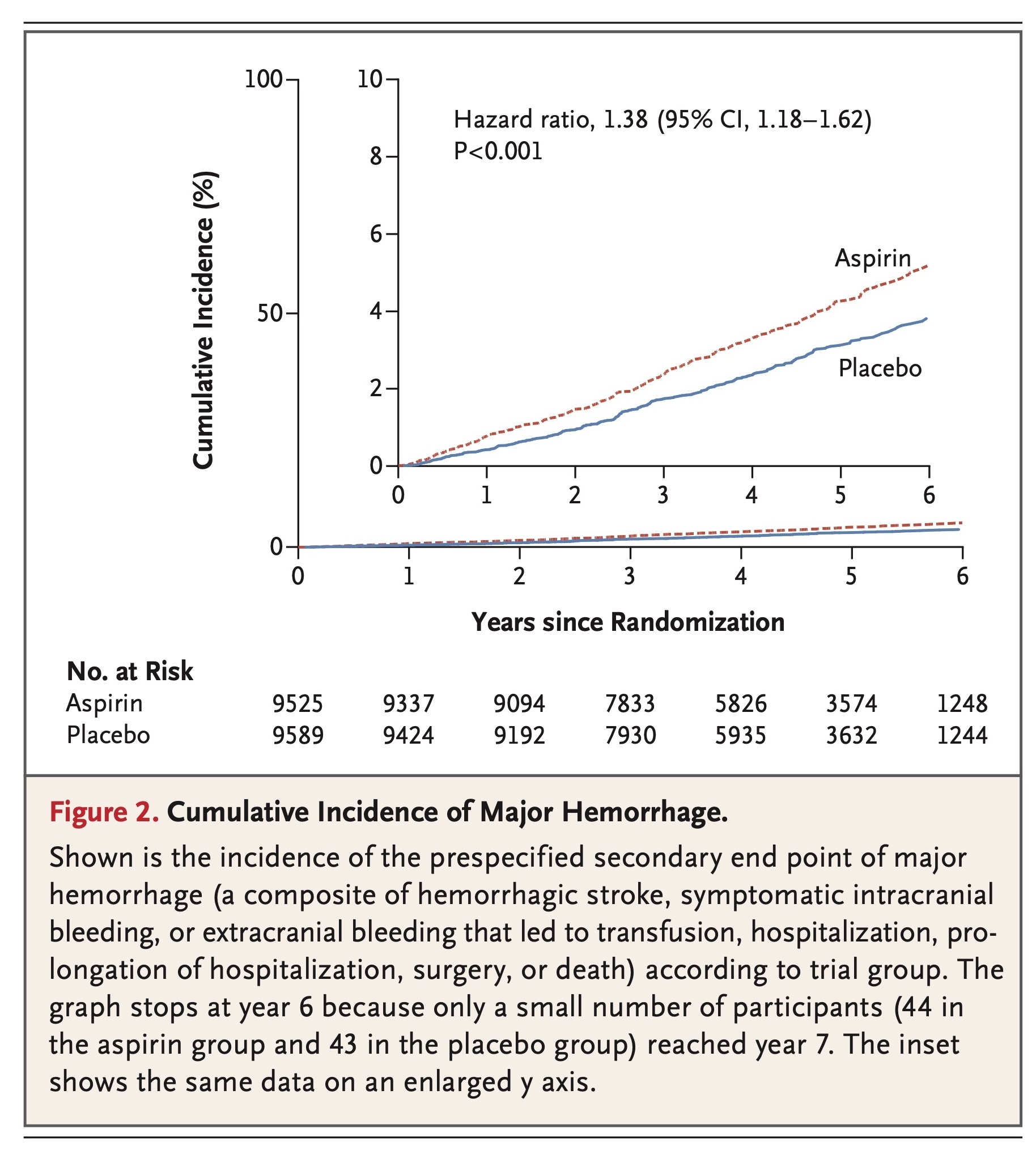
Rates of major hemorrhagic events are shown in Table 3, cumulative incidences are shown in Figure 2, and further details, including data on subclassification of the events, are provided in Table S4 in the Supplementary Appendix. The rate of major hemorrhage was 8.6 events per 1000 person-years in the aspirin group, as compared with 6.2 events per 1000 person-years in the placebo group (hazard ratio, 1.38; 95% CI, 1.18 to 1.62; P<0.001). The progressive increase in the cumulative incidence of major hemorrhage across the trial follow-up period indicates that the risk of bleeding persisted throughout the course of therapy (Figure 2). The rate of fatal hemorrhage was less than 1 event per 1000 person-years in each group.
Gastrointestinal bleeding accounted for just less than half the major hemorrhagic events. The higher risk of upper gastrointestinal bleeding with aspirin than with placebo was particularly pronounced (hazard ratio, 1.87; 95% CI, 1.32 to 2.66) (Table 3). The risk of intracranial bleeding was also higher with aspirin than with placebo (hazard ratio, 1.50; 95% CI, 1.11 to 2.02) (Fig. S9 in the Supplementary Appendix), and this finding was reflected across all subtypes of intracranial bleeding (Table 3). There was no evidence of a differential effect of aspirin on the risk of bleeding in any subgroup analysis, except for the possibility of a less harmful effect with increasing age (Figs. S10 and S11 in the Supplementary Appendix).





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 