J Thromb Haemost. 2019 Jan;17(1):195-205. doi: 10.1111/jth.14338. Epub 2018 Dec 13.
Abstract
Essentials
- Delayed treatment with tranexamic acid results in loss of efficacy and poor outcomes.
- Increasing urokinase activity may account for adverse effects of late tranexamic acid treatment.
- Urokinase + tranexamic acid produces plasmin in plasma or blood and disrupts clotting.
- α2‐Antiplasmin consumption with ongoing fibrinolysis increases plasmin‐induced coagulopathy.
簡單地說,tranexamic acid 是不是有明顯效果是跟出血手上的環境還有時間有關係。
最重要的決定因素有:
1. α2 -antiplasmin 的濃度:也就是我最上面那張示意圖
在病情惡化或是出血繼續的情況下,α2 -antiplasmin 被漸漸消耗,所以之後再給與tranexamic acid也沒有辦法有足夠的資源來結合產生fibrinosis。
2. uPA(urokinase plasminogen activator) 和tranexamic acid會增強plasmin 活化,促進fibrinolysis。
於是在uPA與TXA同時存在的狀況下,tranexamic acid會和plasminogen 以低親和力結合,這個結合會讓plasminogen產生transformation,導致uPA活化plasminogen 成為plasmin 的能力增加,進而更看不見止血的效果。
Summary
Background
Tranexamic acid (TXA) is an effective antifibrinolytic agent with a proven safety record. However, large clinical trials show TXA becomes ineffective or harmful if treatment is delayed beyond 3 h. The mechanism is unknown but urokinase plasminogen activator (uPA) has been implicated.
Methods
Inhibitory mechanisms of TXA were explored in a variety of clot lysis systems using plasma and whole blood. Lysis by tissue plasminogen activator (tPA), uPA and plasmin were investigated. Coagulopathy was investigated using ROTEM and activated partial thromboplastin time (APTT).
Results
IC50 values for antifibrinolytic activity of TXA varied from < 10 to > 1000 μmol L−1depending on the system, but good fibrin protection was observed in the presence of tPA, uPA and plasmin. However, in plasma or blood, active plasmin was generated by TXA + uPA (but not tPA) and coagulopathy developed leading to no or poor clot formation. The extent of coagulopathy was sensitive to available α2‐antiplasmin. No clot formed with plasma containing 40% normal α2‐antiplasmin after short incubation with TXA + uPA. Adding purified α2‐antiplasmin progressively restored clotting. Plasmin could be inhibited by aprotinin, IC50 = 530 nmol L−1, in plasma.
Conclusions
Tranexamic acid protects fibrin but stimulates uPA activity and slows inhibition of plasmin by α2‐antiplasmin. Plasmin proteolytic activity digests fibrinogen and disrupts coagulation, exacerbated when α2‐antiplasmin is consumed by ongoing fibrinolysis. Additional direct inhibition of plasmin by aprotinin may prevent development of coagulopathy and extend the useful time window of TXA treatment.
Introduction
Tranexamic acid (TXA) is a potent antifibrinolytic that has been in common use for many years with an excellent safety record 1-3. As a lysine analogue, TXA binds to kringle domains that have an affinity for lysine residues in proteins, and for fibrinolysis the relevant kringles are in plasminogen and plasmin, and kringle 2 of tissue plasminogen activator (tPA) 4. By blocking plasminogen–fibrin interactions (and to a lesser extent tPA–fibrin interactions 5), TXA inhibits tPA‐catalysed plasmin generation. By binding to plasmin kringles, TXA can inhibit plasmin accumulation by fibrin and directly reduce fibrinolysis 6, 7. Antifibrinolytics, including lysine analogues and the direct plasmin inhibitor aprotinin (Trasylol™), have been employed over many years to limit blood loss and reduce volumes of blood and blood product transfusions in a range of surgical procedures 8, in particular in cardiopulmonary bypass and orthopedic surgery. Lysine analogues can also be taken orally to help in the management of menorrhagia 1, 2, 9 and have been explored as adjuncts to clotting factor replacement therapy to treat hemophilia 10, 11. Antifibrinolytics have been shown to be effective in the treatment of bleeding and reducing mortality 12, particularly in two large trials on trauma (CRASH‐2 13) and post‐partum hemorrhage (WOMAN trial 14). Subsequent subgroup analysis found early treatment in under 3 h is most effective and later treatment can be harmful 15. A recent meta‐analysis of > 40 000 acute severely bleeding patients provided a model indicating each 15 min of delay of TXA administration reduced effectiveness by 10% until there was no benefit after 3 h 16. This analysis also confirmed that TXA treatment did not increase risk of death from vascular occlusive events. However, as these authors pointed out, further research is required on the mechanism of action of TXA to understand the time dependence and risks.
In a controlled surgical setting where TXA can be given before bleeding starts, a wide range of dose regimes are found in different types of surgery, including cardiac, gynecological, liver, orthopedic and neurosurgery 1, 2, 17. Where it has been investigated, effective circulating concentrations of 10–150 μg mL−1 or 60–950 μmol L−1 are quoted to give clinically significant levels of antifibrinolytic activity that reduce blood loss 2. Higher levels of TXA have been reported to cause seizures, which constitute a significant risk factor after surgery 18. However, dosing guidelines for TXA have been developed empirically with little hard supporting evidence, so ideal dose regimens have not been identified.
Better information is needed on effective concentrations of TXA in vivo and how TXA activity in vitro relates to the in vivo situation. With this information it may be possible to optimize TXA administration protocols to get maximum antifibrinolytic activity with lowest risk of side‐effects. Possible mechanisms behind the 3‐h window of effectiveness of TXA observed in the CRASH‐2 and WOMAN trials have been considered 3, but clear evidence is lacking. Generation of plasmin activity by urokinase plasminogen activator (uPA) and TXA is the focus of the current work, which builds on earlier physicochemical and biochemical studies, and animal models. We observe that the potential for proteolytic damage caused by uncontrolled plasmin activity, generated by uPA + TXA, is increased as α2‐antiplasmin is consumed during ongoing fibrinolysis. Targeting unwanted plasmin activity may be a route to extending the useful therapeutic time window of TXA.
Methods
Clot lysis methods
Many methods exist to investigate plasma clot lysis 19 and in this study the procedure of Antovic et al. 20 was the basis of most methods. Briefly, freeze‐dried plasma (NIBSC code 06/158; NIBSC, South Mimms, UK) was reconstituted and 70 μL mixed with 50 μL of buffer (66 mmol L−1 Tris/HCl pH 7.4 containing 130 mmol L−1 NaCl, 45 mmol L−1 CaCl2 and 0.01% Tween 20), containing 0.1 IU mL−1 thrombin (01/578, NIBSC) and plasminogen activator (final concentrations are given in Results). Then 10 μL of TXA (the range of concentrations is shown in Results) and 20 pmol L−1 tissue factor (14/230, NIBSC) were added to the mixture before clotting. Plasminogen activators used were tPA (code 98/714), uPA (11/184), single chain urokinase plasminogen activator (scuPA) (92/714) or plasmin (13/206), all from NIBSC. Euglobulin was prepared following the method of Urano et al. 21 using reconstituted plasma (06/158). Clotting and lysis curves, usually in duplicate, were generated by monitoring absorbance at 405 nm over time and analyzed using online apps 22 to determine time to 50% lysis. Inhibition by TXA was expressed as extension of time to 50% lysis at each TXA concentration compared with no added TXA, which involved the minimum of data manipulation. Data were fitted to a one‐site‐specific binding equation using GraphPad Prism (GraphPad Software, La Jolla, CA, USA) to calculate IC50 values (± standard error [SE] from curve fitting).
To measure release of plasmin from clots, plasma was clotted as described above with incorporation of 2.5 nmol L−1 tPA or 5 nmol L−1 uPA in the clot but without TXA. After 30 min, 10 μL of a mixture of 1.5 mmol L−1 S‐2251 chromogenic substrate (H‐D‐Val‐Leu‐Lys‐pNA; Chromogenix, Milan, Italy) and TXA over the range stated in Results was added to the formed clot and change in absorbance was measured over time. Where present, aprotinin (Baxter, Vienna, Austria) was also added to this solution, over the concentration range shown in Results. Concentrations of aprotinin were expressed in μmol L−1 using a conversion factor of 1.4 mg mL−1 as equivalent to 10 000 Kallikrein Inhibitor Units (KIU) and 215 μmol L−1 (from Baxter product literature). Results were analyzed using an online app 22that determines rates of plasmin generation from chromogenic substrate hydrolysis by calculating slopes of plots of absorbance at 405 nm vs. time squared.
A modification of the halo method of Bonnard et al. 23 was used to compare TXA inhibition of fibrinolysis in whole blood. Briefly, a drop of blood is mixed with clotting solution and smeared as a “halo” around the base of a well in a microtitre plate, leaving the center of the well clear. As lysis takes place, absorbance of the solution in the well increases and is monitored. Clots were made as described previously 23, except 15 μL of blood was clotted with 5 μL of the clotting mixture described above for clot lysis assays 20 and clotting was allowed to proceed for 30 min. Mixtures, 80 μL of uPA or tPA with TXA at the concentrations stated in Results, were added to initiate clot lysis. This assay was also modified to use plasma in place of blood. Freeze‐dried plasma (06/158, NIBSC) was reconstituted in water and to this was added fluorescently labelled fibrinogen (Alexa Fluor‐488; Thermofisher Scientific, Waltham, MA, USA) to a final concentration of 0.15 mg mL−1. Lysis was monitored by release of fluorescently labelled fibrin degradation products from the halo clot. Data were analyzed using an online app specifically designed to analyze halo assay data and provide values for times to 50% clot lysis with either blood or plasma clots 24. To investigate plasmin generation from plasma clots the same method was used as described above, but without addition of fluorescent fibrinogen. Plasmin chromogenic substrate S‐2251 (1.5 mmol L−1final concentration) was included in the mixture of TXA and plasminogen activator.
ROTEM delta (Werfen, Warrington, UK) equipment was operated according to the manufacturer's instructions using Intem reagents supplied by the manufacturer. Blood was collected from local donors, with approval of the local ethical committee. Activated partial prothrombin time (APTT) of plasma was determined using a KC4 Delta semiautomated coagulation analyzer supplied by Trinity Biotech (Bray, Co Wicklow, Ireland). APTT‐SP reagents were from Instrumentation Laboratory (Milan, Italy) and were used according to the manufacturer's instructions. Plasma used for APTT determinations was freeze dried (06/158, NIBSC) or was fresh frozen therapeutic plasma (V.I. plasma) that had been treated with solvent detergent (Octaplas LG, Octapharma, Stockholm, Sweden). In some cases, V.I. plasma was supplemented with purified, plasma‐derived α2‐antiplasmin (Merck, Nottingham, UK). Plasmin digestion of fibrinogen was identified by SDS PAGE and Coomassie staining using 4–12% Bis‐Tris Plus gels. Where V.I. plasma was used, fibrinogen digestion was followed by western blotting using a polyclonal rabbit anti‐human fibrinogen antibody (A0030; Dako, Glostrup, Denmark).
Results
The effective range of TXA in vitro
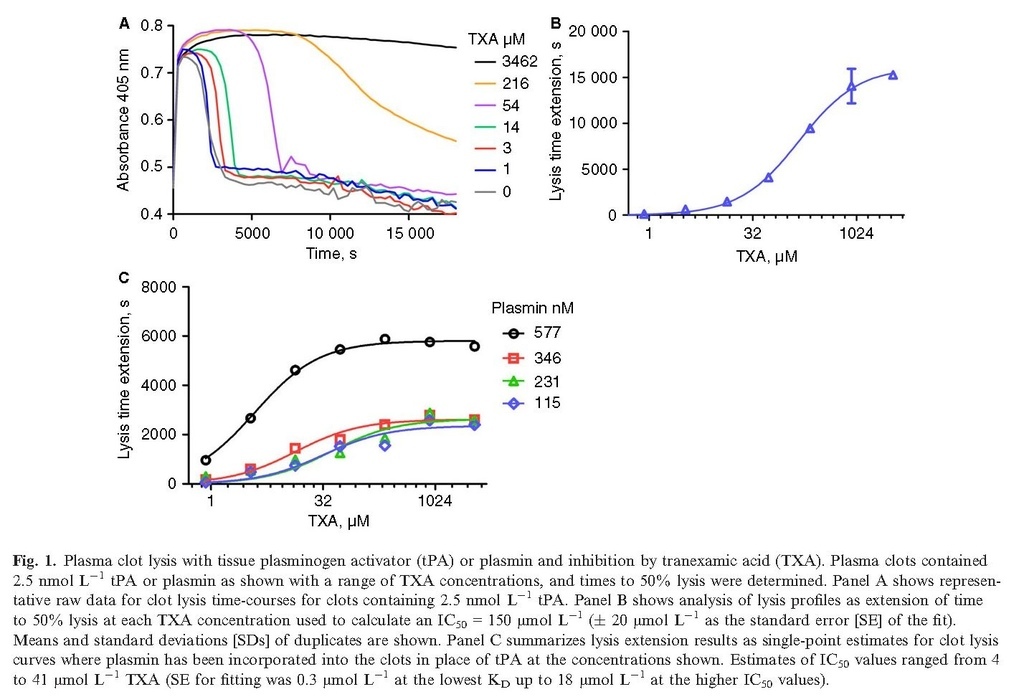
In purified systems of fibrinolysis, kinetic models previously gave estimates of KD < 10 and 30 μmol L−1 for glu‐ and lys‐plasminogen, respectively 5. The observed values for IC50 in more complex experimental systems will be affected by other components such as higher plasminogen concentrations, the presence of fibrin and α2‐antiplasmin. In a plasma clot lysis system an IC50 = 150 (± 20) μmol L−1 for TXA was observed, as shown in Fig. 1(A,B), using 2.5 nmol L−1 tPA as activator. In this system, uPA or scuPA (data not shown) were less effective alone as an activator than tPA and no fibrinolysis was seen with 10 nmol L−1 uPA or scuPA as sole activator, over the 5‐h time‐courses used in these experiments. Plasmin incorporated into clots during clotting was inhibited by TXA, as shown in Fig. 1(C). Plasmin concentrations in the range of 115–577 nmol L−1 were sensitive to TXA and IC50 values were 4–41 μmol L−1.
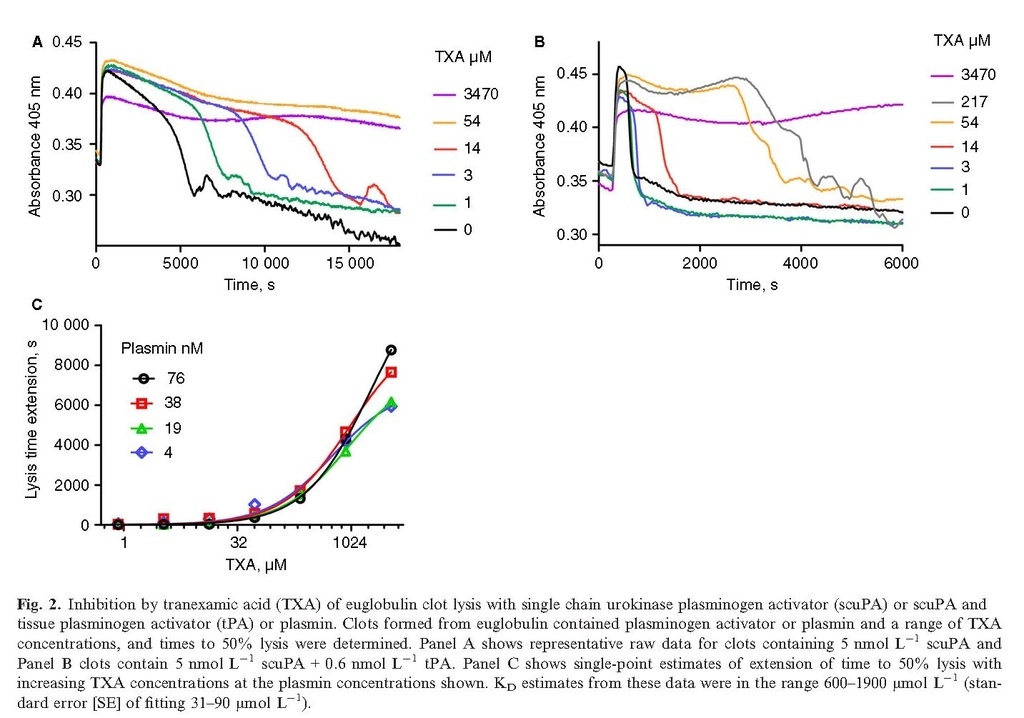
Plasma clots are stabilized by α2‐antiplasmin, but in situations of ongoing fibrinolysis α2‐antiplasmin may be depleted and the system will be more susceptible to fibrinolysis. A series of experiments were performed using clots made from euglobulin, which is known to have reduced levels of inhibitors, including α2‐antiplasmin 25. Representative clot lysis profiles are shown in Fig. 2, where 5 nmol L−1 scuPA, the single chain zymogen of uPA, could catalyze fibrinolysis. Marked stimulation of fibrinolysis was achieved by adding 0.6 nmol L−1 tPA to scuPA (Fig. 2B, note the shorter time on the x axis). Using euglobulin as substrate, the speed of fibrinolysis correlated with the observed IC50 for TXA blocking fibrinolysis. For example, for 5 and 10 nmol L−1 scuPA alone, estimates of IC50 values were 13 (± 3) and 214 (± 90) μmol L−1, and with the addition of 0.6 nmol L−1 tPA the IC50 values were 380 (± 144) and 490 (± 231) μmol L−1 (± values are SE for curve fitting). Euglobulin clots were more sensitive to direct application of plasmin compared with plasma clots, as shown in Fig. 2(C), and IC50 vales for TXA were high, estimated to be 600–1900 (± 31–90) μmol L−1. Thus, reducing available intrinsic inhibitors of fibrinolysis increased the concentration of TXA required to block fibrinolysis.
Halo and ROTEM methods
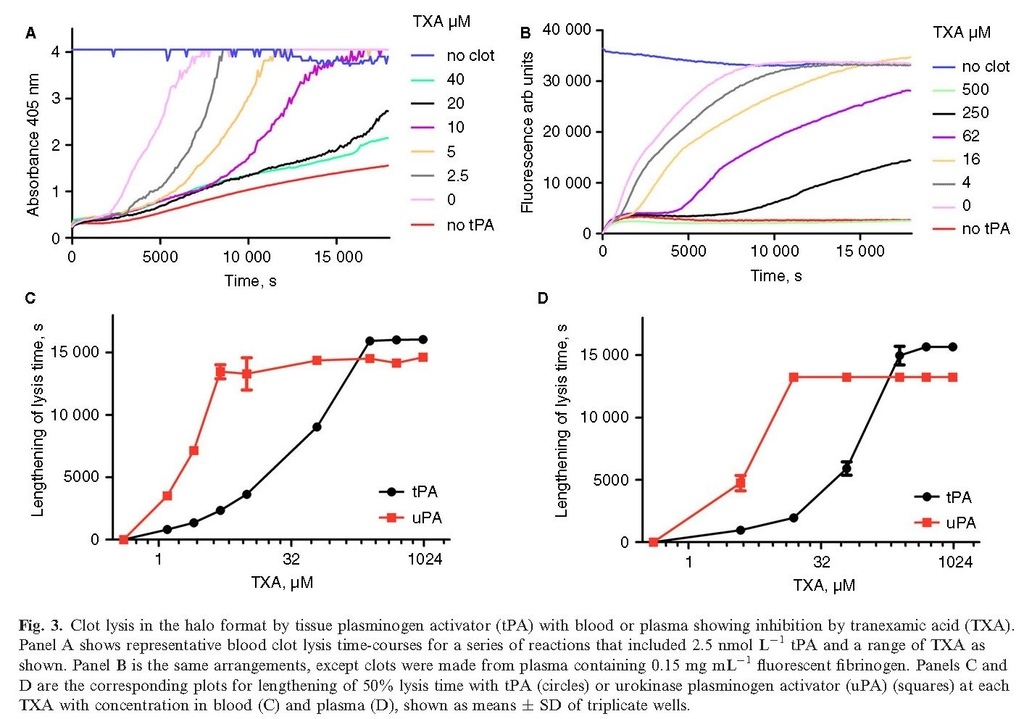
The recently described halo method 23 is a microtiter plate‐based method to measure fibrinolysis of clots made from whole blood. We have adapted the method to investigate inhibition by TXA and also modified it by adding fluorescent fibrinogen to plasma before clotting to investigate plasma clot lysis under the same conditions. Figure 3(A,B) shows inhibition by a range of TXA with 2.5 nmol L−1 tPA using the halo format. IC50 values (± SE of fitting) were 44 (± 4) and 102 (± 16) μmol L−1 TXA for blood or plasma clots, respectively. Lysis by 10 nmol L−1 uPA was more sensitive to TXA and estimates for IC50 values were 2–5 μmol L−1 TXA (see Fig. 3C,D). One significant difference between the microtiter plate formats shown in Figs 1 and 3 is the amount of fibrin present in the wells. In our version of the halo assay only 15 μL of blood or plasma is used, whereas in the clot lysis system used in Figs 1and 2, 70 μL of plasma or euglobulin is used. These factors may contribute to the differences in effectiveness of TXA in the two microtiter plate methods.
ROTEM experiments were performed using platelet‐poor and platelet‐rich plasma and whole blood with added tPA or uPA and TXA. Results were consistent across different substrates. The range of activators explored was between 2 and 10 nmol L−1 for both tPA and uPA, and TXA down to 10 μmol L−1 was able to protect clots from lysis during the timescales of the ROTEM experiments, which is up to 1 h (data not shown).
Plasmin generation by uPA and TXA
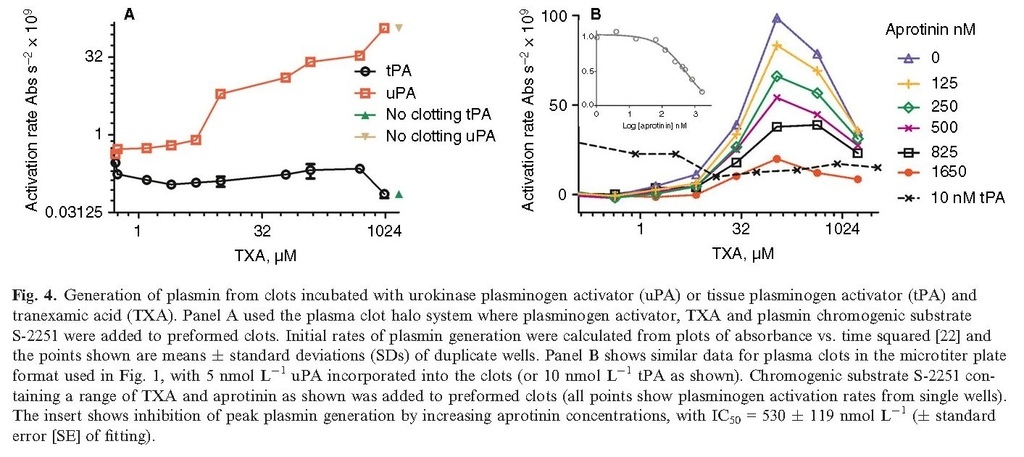
All results described above show that TXA can protect fibrin from breakdown in a variety of formats when either tPA or uPA is activator or with directly applied plasmin. By adjusting the methods used in Figs 1 and 3 so that plasmin chromogenic substrate was added to preformed clots along with uPA or tPA, it was possible to detect the generation of plasmin released from the clot. Figure 4(A) shows results from the halo/plasma clot system (no added Alexa Fluor fibrinogen in this case) and Fig. 4(B) shows results from the other microtiter plate plasma clot lysis system (shown in Fig. 1), now including S‐2251. In both formats, active plasmin was released by uPA, but not tPA, in the presence of TXA. Stimulation begins above 10 μmol L−1 TXA and reaches a peak at around 100 μmol L−1, followed by a decline as shown in Fig. 4(B), but this decline is not observed in the halo format, Fig 4(A). Fig. 4(B) also shows that the plasmin inhibitor aprotinin can block plasmin activity generated by uPA and TXA. Calculations on the dose response of aprotinin gave an estimated IC50 of 530 (± 119) nmol L−1 (see Fig. 4B inset).
Free plasmin disrupts coagulation and can break down fibrinogen in the presence of TXA
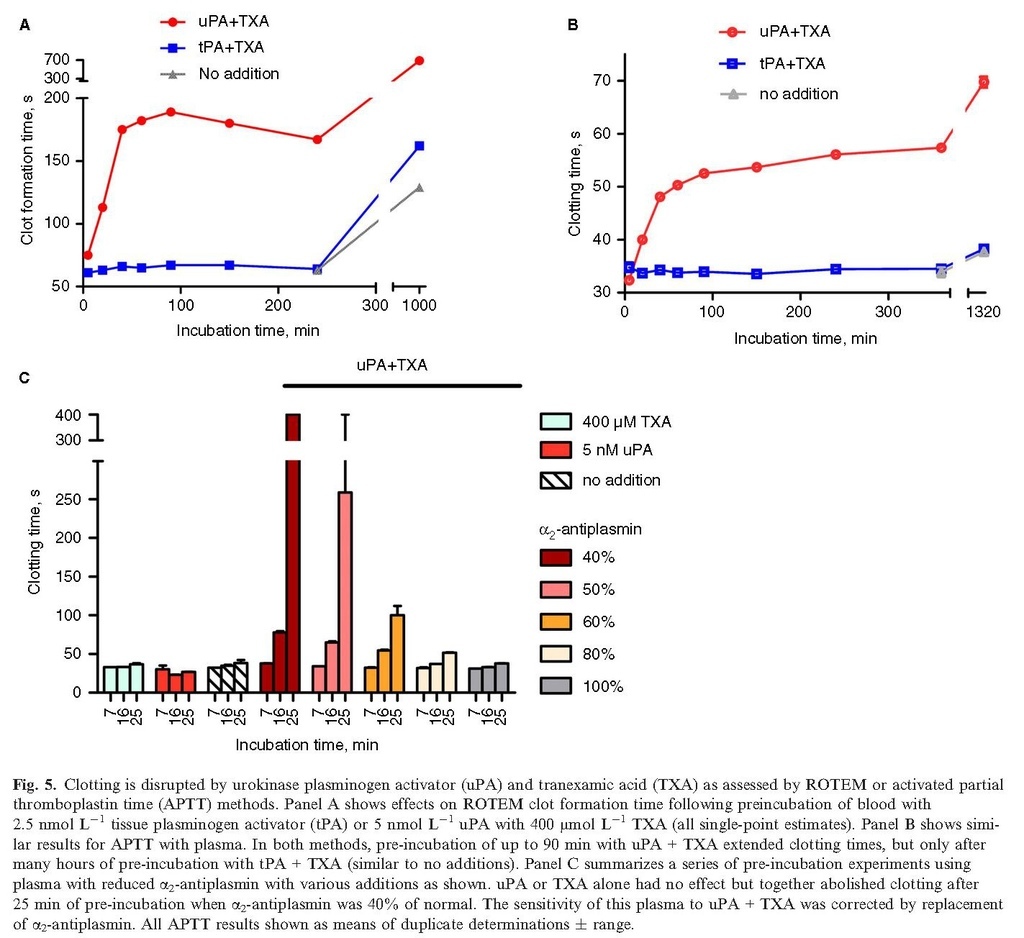
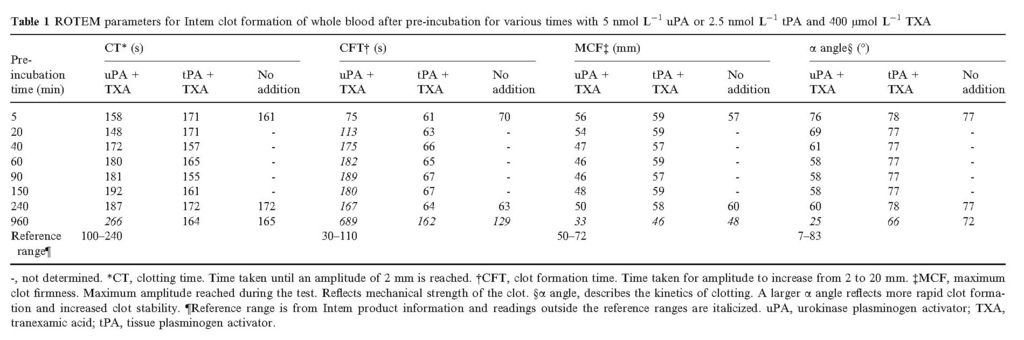
Having demonstrated that uPA + TXA can generate plasmin activity in plasma, the consequences of this proteolytic activity for coagulation were explored. Disruption of coagulation was observed in whole blood and plasma in studies using ROTEM and APTT assay systems, as summarized in Fig. 5. Many of the clotting parameters determined in ROTEM studies were affected by pre‐incubations of 400 μmol L−1 TXA and 5 nmol L−1 uPA, but not with TXA and 2.5 nmol L−1 tPA (before overnight incubation), as indicated in Table 1and Fig. 5(A) for clot formation time (CFT). Initiation of clotting (CT) was less affected by pre‐incubation with uPA + TXA than other parameters that describe clot quality (CFT, maximum clot firmness (MCF) and α‐angle; see Table 1 for details of these parameters). In agreement with ROTEM results, extended plasma APTT values were observed in the presence of 5 nmol L−1 uPA and 400 μmol L−1 TXA, but not tPA, as shown in Fig. 5(B). The importance of α2‐antiplasmin in suppressing liberated plasmin activity is illustrated in Fig. 5(C) using treated plasma with a reduced concentration of active α2‐antiplasmin 26. In this case, α2‐antiplasmin was 0.4 U mL−1, or 40% of normal (other coagulation factors, V, VIII and XI, were 1.0–1.1 U mL−1, fibrinogen was 3.2 mg mL−1 and APTT was 28–32 s before any additions or pre‐incubations). Clotting was seriously impaired by low α2‐antiplasmin, so at 0.4 U mL−1 no clot formed after 25 min incubation with 5 nmol L−1 uPA and 400 μmol L−1 TXA. Stepwise normalization of clotting over these short incubation times was achieved with addition of purified α2‐antiplasmin to 50, 60, 80 and 100% of normal levels, as shown in Fig 5(C).
Table 1. ROTEM parameters for Intem clot formation of whole blood after pre‐incubation for various times with 5 nmol L−1 uPA or 2.5 nmol L−1 tPA and 400 μmol L−1 TXA
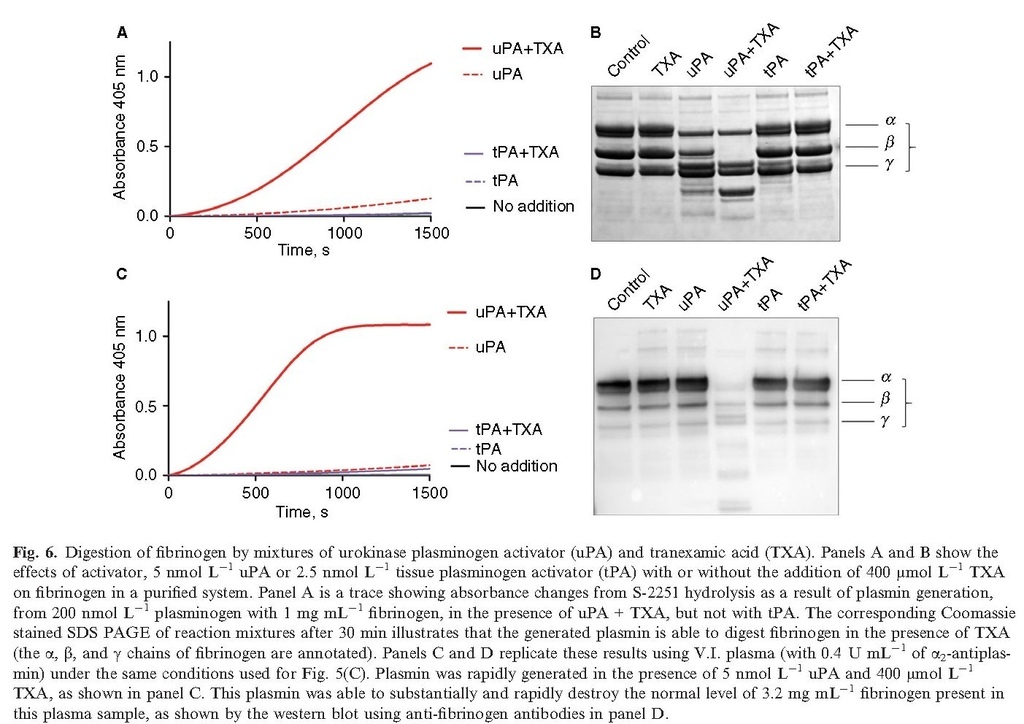
Fibrinogen degradation resulting from plasmin generation is likely to contribute to disrupted coagulation (although other factors may also be proteolyzed), as shown in Fig. 6. Mixtures of 5 nmol L−1 uPA and 400 μmol L−1 TXA are able to rapidly generate free plasmin in a purified system containing fibrinogen and plasminogen, or in the same V.I. plasma as used in Fig. 5(Fig. 6 panels A and C, respectively). The corresponding Coomassie‐stained SDS PAGE or western blot (B and D, respectively) illustrates the plasmin digestion of fibrinogen after 30 min of incubation with uPA + TXA. The corresponding results using normal plasma are shown in supplementary Figure S1. However, fibrinogen may not be the only target of plasmin generated by uPA + TXA and clotting may be further disrupted by proteolytic attack on other coagulation factors.
Discussion
The loss of effectiveness of TXA with treatment delay is established in meta‐analysis of clinical trials 16, but the mechanism is unknown. A role for uPA has been suggested previously 5, 27, 28 and several possible mechanisms may be involved. Firstly, TXA (or other lysine analogues) has long been known to interact with a low‐affinity plasminogen kringle binding site, KD around 200–600 μmol L−1, to induce a large conformational change, accelerating plasmin generation catalyzed by uPA 29-31. tPA or tPA serine protease domain is less sensitive to this plasminogen conformational change 5, 32. Secondly, TXA protects plasmin from inhibition by α2‐antiplasmin by slowing down the rate of inhibition, with effects observed from 10 to 500 μmol L−1 TXA 33, 34. According to detailed structural and kinetic work 35, the lysine‐rich C‐terminal peptide of α2‐antiplasmin from Asn‐410 to Lys‐464 regulates initial complex formation with plasmin, and its removal was found to reduce the rate of complex formation 40‐fold, as did the presence of 1 mmol L−1 aminohexanoic acid. A third line of evidence for a potential role for uPA in trauma comes from animal models. A model of traumatic brain injury in mice revealed a rapid spike of tPA release up to 12 nmol L−1 measured in cerebrospinal fluid, 1 h after injury, which was followed by a slow release of uPA, peaking at 8 nmol L−1, 8 h after injury (mirrored by lower concentrations of tPA and uPA in blood) 27. The release of plasminogen activators was associated with intracranial hemorrhage, which increased if TXA was administered 8 h after the traumatic event, as uPA levels peaked. A model of cerebral hypoxia/ischemia in pigs also showed increases in uPA concentrations in cerebrospinal fluid after injury, up to 2.5 nmol L−1, 4 h post‐injury 36. The authors concluded that tPA is initially released from stores, but uPA is synthesized de novoby brain cells.
In studies on trauma patients, limited information is available on changes in uPA, with much more focus on tPA, PAI‐1, D‐dimer and plasmin‐ α2‐antiplasmin (PAP) complexes. Normally, there is no active uPA in the circulation, and single chain, inactive scuPA is present at 2–4 ng mL−1 or around 10−10 M 37, although higher concentrations are measured in cancer patients. However, some trauma patients show increased levels of uPA over time 38 or in the most severe cases 39, but correlations with poor survival have not been demonstrated. The dangers of disturbed fibrinolysis in trauma‐induced coagulopathy are accepted, and hyperfibrinolysis, which is present in a minority of patients, represents a major risk factor for early death. For example, Raza and co‐workers investigated fibrinolysis proteins in patients demonstrating normal, moderate and severe hyperfibrinolysis 40. Significant findings in this study included a 5‐fold rise in tPA concentrations to 0.6 nmol L−1, along with 30–40% decreases in circulating α2‐antiplasmin and fibrinogen in severely affected patients. PAP increased around 19‐fold, emphasizing the significant generation of plasmin in these patients with parallel consumption of α2‐antiplasmin. These snapshots do not provide a complete picture of how fibrinolysis and coagulation proteins can change over time or what effects TXA would have. In the current study, the risk of coagulopathy associated with depletion of endogenous α2‐antiplasmin and the presence of uPA + TXA is highlighted in Figs 5 and 6. Furthermore, results in Fig. 2 suggest scuPA in combination with tPA, as expected in vivo, is much more potent than scuPA alone. The potential to generate free plasmin and the development of overt hyperfibrinolysis in trauma is likely to be regulated by increased concentrations of tPA and scuPA, alongside decreased α2‐antiplasmin and fibrinogen concentrations. The concentrations of tPA and uPA used in the current study were selected to rapidly initiate fibrinolysis in normal plasma or blood. Initial studies suggest much lower concentrations of tPA and (sc)uPA are necessary to produce rapid plasmin generation when α2‐antiplasmin is depleted, but further work is needed to explore these concentration dependencies in detail.
The data presented in Fig. 4 illustrate generation of free plasmin activity by TXA + uPA, and the potential of aprotinin to substitute for α2‐antiplasmin and block this activity in plasma. The concentrations of aprotinin required to control plasmin activity were quite high, > 500 nmol L−1, in this assay system, in the context of the high affinity of plasmin for aprotinin (KDaround 2 nmol L−1) 41. However, aprotinin up to 3 μmol L−1 has been used in high dose regimes in cardiac surgery 42, 43.
The optimum concentration of TXA
Tranexamic acid is able to protect fibrin from degradation by plasmin, but the effective concentration and IC50 in vitro is variable. Protection is seen where tPA or uPA is activator or where plasmin is applied directly to clots, supporting a mechanism where kringle‐dependent plasminogen and plasmin binding is blocked by TXA to inhibit fibrinolysis. Direct application of plasmin resulted in the lowest TXA IC50 values (4–41 μmol L−1) using plasma clots (Fig. 1C) and the highest IC50 values in euglobulin clots (600–1900 μmol L−1). These differences could be dependent on several factors, including the amount of plasmin or plasminogen or α2‐antiplasmin available. Fibrinolysis in the ROTEM system was sensitive to TXA as low as 10 μmol L−1, even using whole blood. A factor here could be that ROTEM monitors the early stages of fibrinolysis, at a time where plasmin only begins to develop, which may reduce the requirement for TXA. The results highlight the variability inherent in different experimental set‐ups. In a purified system, we previously estimated the KD of TXA for plasminogen that blocked plasminogen activation by tPA or uPA to be around 10 μmol L−1 or less for glu‐plasminogen, or 30 μmol L−1 for lys‐plasminogen 5. Therapeutic concentrations of TXA used in cardiopulmonary bypass and other elective surgical procedures that are easier to control than traumatic bleeding, aim for TXA in circulation around 100 μmol L−1 or higher 1, 2, 8. Considering all our results, it seems unlikely there will be an ideal concentration that could provide adequate fibrin protection in all situations in vivo but be insufficient to induce the plasminogen conformational change (above 100 μmol L−1) that enhances uPA activity.
Trauma‐induced coagulopathy, disseminated intravascular coagulation and fibrinolysis shutdown
Disseminated intravascular coagulation (DIC) may be categorized as being a hemorrhagic or thrombotic phenotype 44-46. Guidelines for the diagnosis of DIC, such as those prepared by the ISTH/SSC, rely on a scoring system for low platelet count, low fibrinogen, prolonged clotting time and elevated D‐dimer (e.g. see 47, 48). However, anatomopathologic diagnosis of DIC, seen as widespread accumulation of microthrombi, is also viewed as the diagnostic reference standard for thrombotic DIC 49. The accumulation and consequences of microthrombi in trauma are proposed as a mechanism to explain the observed increased risk of delayed TXA administration. According to this theory, early stages of trauma, involving bleeding with rapid release of tPA and increased fibrinolysis, are likened to DIC with a hemorrhagic phenotype. Subsequently, a thrombotic phenotype may develop, associated with increased PAI‐1, leading to suppressed fibrinolysis, increasing the risk of accumulation of microvascular thrombi, organ failure and death. 46. This risk could be further increased by late treatment with TXA. However, it is noteworthy that meta‐analysis of clinical trials of TXA in bleeding patients generally does not find increased risk of vascular occlusive events 16, and TXA has an excellent safety record across a range of applications despite its capacity to efficiently shut down fibrinolysis. Furthermore, in one detailed study of organs recovered from autopsies of severely injured trauma patients, no pathologic evidence of microthrombi in small and mid‐size vessels could be found 49. Conclusive evidence is lacking for delayed fibrinolysis shutdown being responsible for the failure of TXA to improve mortality when given after 3 h.
Conclusions
We hypothesize that uPA and α2‐antiplasmin are key factors in the mechanism accounting for loss of efficacy of TXA in controlling bleeding after treatment delay. Hemorrhage associated with trauma, childbirth and surgical interventions will lead to activation of clotting and fibrinolysis and generation of tPA and uPA and subsequently plasmin. Importantly, ongoing fibrinolysis causes depletion of α2‐antiplasmin. Application of TXA is very effective at protecting fibrin, but TXA can stimulate plasminogen activation by uPA and protect plasmin in circulation from α2‐antiplasmin. Free plasmin can induce coagulopathy by proteolyzing coagulation factors, including fibrinogen (50 and Fig. 6), FV and FVIII 51-53 and FXIII 54 and can damage the blood–brain barrier 55. At the stage where there is overt hyperfibrinolysis, direct inhibition of plasmin by exogenous inhibitors may be necessary. However, more information is needed on the time‐course and magnitude of uPA production in trauma patients, particularly the minority that develop life‐threatening hyperfibrinolysis.
Preference
- 1Ng W, Jerath A, Wasowicz M. Tranexamic acid: a clinical review. Anaesthesiol Intensive Ther 2015; 47: 339–50.
- 2Tengborn L, Blomback M, Berntorp E. Tranexamic acid – an old drug still going strong and making a revival. Thromb Res 2015; 135: 231–42.
- 3Kolev K, Longstaff C. Bleeding related to disturbed fibrinolysis. Br J Haematol 2016; 175: 12–23.
- 4Longstaff C, Kolev K. Basic mechanisms and regulation of fibrinolysis. J Thromb Haemost 2015; 13(Suppl. 1): S98–105.
- 5Silva MM, Thelwell C, Williams SC, Longstaff C. Regulation of fibrinolysis by C‐terminal lysines operates through plasminogen and plasmin but not tissue plasminogen activator (tPA). J Thromb Haemost 2012; 10: 2354–60.
- 6Varju I, Tenekedjiev K, Keresztes Z, Pap AE, Szabo L, Thelwell C, Longstaff C, Machovich R, Kolev K. Fractal kinetic behavior of plasmin on the surface of fibrin meshwork. Biochemistry 2014; 53: 6348–56.
- 7Kolev K, Komorowicz E, Owen WG, Machovich R. Quantitative comparison of fibrin degradation with plasmin, miniplasmin, neurophil leukocyte elastase and cathepsin G. Thromb Haemost 1996; 75: 140–6.
- 8Ker K, Prieto‐Merino D, Roberts I. Systematic review, meta‐analysis and meta‐regression of the effect of tranexamic acid on surgical blood loss. Br J Surg 2013; 100: 1271–9.
- 9Lumsden MA, Wedisinghe L. Tranexamic acid therapy for heavy menstrual bleeding. Expert Opin Pharmacother 2011; 12: 2089–95.
- 10Forbes CD, Barr RD, Reid G, Thomson C, Prentice CR, McNicol GP, Douglas AS. Tranexamic acid in control of haemorrhage after dental extraction in haemophilia and Christmas disease. BMJ1972; 2: 311–3.
- 11Hvas AM, Sorensen HT, Norengaard L, Christiansen K, Ingerslev J, Sorensen B. Tranexamic acid combined with recombinant factor VIII increases clot resistance to accelerated fibrinolysis in severe hemophilia A. J Thromb Haemost 2007; 5: 2408–14.
- 12Spahn DR, Bouillon B, Cerny V, Coats TJ, Duranteau J, Fernandez‐Mondejar E, Filipescu D, Hunt BJ, Komadina R, Nardi G, Neugebauer E, Ozier Y, Riddez L, Schultz A, Vincent JL, Rossaint R. Management of bleeding and coagulopathy following major trauma: an updated European guideline. Crit Care 2013; 17: R76.
- 13Shakur H, Roberts I, Bautista R, Caballero J, Coats T, Dewan Y, El‐Sayed H, Gogichaishvili T, Gupta S, Herrera J, Hunt B, Iribhogbe P, Izurieta M, Khamis H, Komolafe E, Marrero MA, Mejia‐Mantilla J, Miranda J, Morales C, Olaomi O, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH‐2): a randomised, placebo‐controlled trial. Lancet 2010; 376: 23–32.
- 14Shakur H, Roberts I, Fawole B, Chaudhri R, El‐Sheikh M, Akintan A, Qureshi Z, Kidanto H, Vwalika B, Abdulkadir A, Etuk S, Noor S, Asonganyi E, Alfirevic Z, Beaumont D, Ronsmans C, Arulkumaran S, Grant A, Afsana K, Gülmezoglu M, et al. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post‐partum haemorrhage (WOMAN): an international, randomised, double‐blind, placebo‐controlled trial. Lancet 2017; 389: 2105–16.
- 15Roberts I, Shakur H, Afolabi A, Brohi K, Coats T, Dewan Y, Gando S, Guyatt G, Hunt BJ, Morales C, Perel P, Prieto‐Merino D, Woolley T. The importance of early treatment with tranexamic acid in bleeding trauma patients: an exploratory analysis of the CRASH‐2 randomised controlled trial. Lancet 2011; 377: 1096–101, 101 e1‐2.
- 16Gayet‐Ageron A, Prieto‐Merino D, Ker K, Shakur H, Ageron FX, Roberts I; Antifibrinolytic Trials Collaboration. Effect of treatment delay on the effectiveness and safety of antifibrinolytics in acute severe haemorrhage: a meta‐analysis of individual patient‐level data from 40 138 bleeding patients. Lancet 2018; 391: 125–32.
- 17Ker K, Edwards P, Perel P, Shakur H, Roberts I. Effect of tranexamic acid on surgical bleeding: systematic review and cumulative meta‐analysis. BMJ 2012; 344: e3054.
- 18Sharma V, Katznelson R, Jerath A, Garrido‐Olivares L, Carroll J, Rao V, Wasowicz M, Djaiani G. The association between tranexamic acid and convulsive seizures after cardiac surgery: a multivariate analysis in 11 529 patients. Anaesthesia 2014; 69: 124–30.
- 19Longstaff C. Measuring fibrinolysis: from research to routine diagnostic assays. J Thromb Haemost 2018; 16: 652–662.
- 20Antovic A, Blombäck M, Sten‐Linder M, Petrini P, Holmström M, He S. Identifying hypocoagulable states with a modified global assay of overall haemostasis potential in plasma. Blood Coagul Fibrinolysis 2005; 16: 585–96.
- 21Urano T, Nishikawa T, Nagai N, Takada Y, Takada A. Amounts of tPA and PAI‐1 in the euglobulin fraction obtained at different pH: their relation to the euglobulin clot lysis time. Thromb Res 1997; 88: 75–80.
- 22Longstaff C; subcommittee on fibrinolysis. Development of Shiny app tools to simplify and standardize the analysis of hemostasis assay data: communication from the SSC of the ISTH. J Thromb Haemost 2017; 15: 1044–6.
- 23Bonnard T, Law LS, Tennant Z, Hagemeyer CE. Development and validation of a high throughput whole blood thrombolysis plate assay. Sci Rep 2017; 7: 2346.
- 24Longstaff C. A Shiny app to analyse halo assay data. version 0.35. https://drclongstaff.shinyapps.io/HalolysisCL/. Accessed 20 September 2018.
- 25Hellstern P. Solvent/detergent‐treated plasma: composition, efficacy, and safety. Curr Opin Hematol 2004; 11: 346–50.
- 26Burnouf T, Goubran HA, Radosevich M, Sayed MA, Gorgy G, El‐Ekiaby M. Impact of Triton X‐100 on alpha 2‐antiplasmin (SERPINF2) activity in solvent/detergent‐treated plasma. Biologicals2007; 35: 349–53.
- 27Hijazi N, Abu Fanne R, Abramovitch R, Yarovoi S, Higazi M, Abdeen S, Basheer M, Maraga E, Cines DB, Higazi AA. Endogenous plasminogen activators mediate progressive intracerebral hemorrhage after traumatic brain injury in mice. Blood 2015; 125: 2558–67.
- 28Medcalf RL. The traumatic side of fibrinolysis. Blood 2015; 125: 2457–8.
- 29Mangel WF, Lin BH, Ramakrishnan V. Characterization of an extremely large, ligand‐induced conformational change in plasminogen. Science 1990; 248: 69–73.
- 30Takada A, Makino Y, Takada Y. Effects of tranexamic acid on fibrinolysis, fibrinogenolysis and amidolysis. Thromb Res 1986; 42: 39–47.
- 31Violand BN, Byrne R, Castellino FJ. The effect of alpha‐, omega‐amino acids on human plasminogen structure and activation. J Biol Chem 1978; 253: 5395–401.
- 32Sinniger V, Merton RE, Fabregas P, Felez J, Longstaff C. Regulation of tissue plasminogen activator activity by cells. Domains responsible for binding and mechanism of stimulation. J Biol Chem 1999; 274: 12414–22.
- 33Longstaff C, Gaffney PJ. Studies on the mechanism of binding of serpins and serine proteases. Blood Coagul Fibrinolysis 1992; 3: 89–97.
- 34Longstaff C, Gaffney PJ. Serpin‐serine protease binding kinetics: alpha 2‐antiplasmin as a model inhibitor. Biochemistry 1991; 30: 979–86.
- 35Lu BG, Sofian T, Law RH, Coughlin PB, Horvath AJ. Contribution of conserved lysine residues in the alpha2‐antiplasmin C terminus to plasmin binding and inhibition. J Biol Chem 2011; 286: 24544–52.
- 36Armstead WM, Cines DB, Bdeir K, Kulikovskaya I, Stein SC, Higazi AA. uPA impairs cerebrovasodilation after hypoxia/ischemia through LRP and ERK MAPK. Brain Res 2008; 1231: 121–31.
- 37Mutch N, Booth NA. Plasmin‐Antiplasmin System. Switzerland: Springer, 2016.
- 38Karri J, Cardenas JC, Matijevic N, Wang YW, Choi S, Zhu L, Cotton BA, Kitagawa R, Holcomb JB, Wade CE. Early fibrinolysis associated with hemorrhagic progression following traumatic brain injury. Shock 2017; 48: 644–50.
- 39Cardenas JC, Matijevic N, Baer LA, Holcomb JB, Cotton BA, Wade CE. Elevated tissue plasminogen activator and reduced plasminogen activator inhibitor promote hyperfibrinolysis in trauma patients. Shock 2014; 41: 514–21.
- 40Raza I, Davenport R, Rourke C, Platton S, Manson J, Spoors C, Khan S, De'Ath HD, Allard S, Hart DP, Pasi KJ, Hunt BJ, Stanworth S, MacCallum PK, Brohi K. The incidence and magnitude of fibrinolytic activation in trauma patients. J Thromb Haemost 2013; 11: 307–14.
- 41Longstaff C. Studies on the mechanisms of action of aprotinin and tranexamic acid as plasmin inhibitors and antifibrinolytic agents. Blood Coagul Fibrinolysis 1994; 5: 537–42.
- 42Henry DA, Carless PA, Moxey AJ, O'Connell D, Stokes BJ, Fergusson DA, Ker K. Anti‐fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev 2011;(3):CD001886.
- 43van Oeveren W, Jansen NJ, Bidstrup BP, Royston D, Westaby S, Neuhof H, Wildevuur CR. Effects of aprotinin on hemostatic mechanisms during cardiopulmonary bypass. Ann Thorac Surg1987; 44: 640–5.
- 44Chang R, Cardenas JC, Wade CE, Holcomb JB. Advances in the understanding of trauma‐induced coagulopathy. Blood 2016; 128: 1043–9.
- 45Frith D, Brohi K. The pathophysiology of trauma‐induced coagulopathy. Curr Opin Crit Care2012; 18: 631–6.
- 46Walsh M, Shreve J, Thomas S, Moore E, Moore H, Hake D, Pohlman T, Davis P, Ploplis V, Piscoya A, Wegner J, Bryant J, Crepinsek A, Lantry J, Sheppard F, Castellino F. Fibrinolysis in trauma: “myth,” “reality,” or “something in between”. Semin Thromb Hemost 2017; 43: 200–12.
- 47Hunt BJ. Bleeding and coagulopathies in critical care. N Engl J Med 2014; 370: 847–59.
- 48Gando S, Levi M, Toh CH. Disseminated intravascular coagulation. Nat Rev Dis Primers 2016; 2: 16037.
- 49Rizoli S, Nascimento BJ, Key N, Tien HC, Muraca S, Pinto R, Khalifa M, Plotkin A, Callum J. Disseminated intravascular coagulopathy in the first 24 hours after trauma: the association between ISTH score and anatomopathologic evidence. J Trauma Acute Care Surg 2011; 71: S441–7.
- 50Rourke C, Curry N, Khan S, Taylor R, Raza I, Davenport R, Stanworth S, Brohi K. Fibrinogen levels during trauma hemorrhage, response to replacement therapy, and association with patient outcomes. J Thromb Haemost 2012; 10: 1342–51.
- 51Nogami K, Shima M, Matsumoto T, Nishiya K, Tanaka I, Yoshioka A. Mechanisms of plasmin‐catalyzed inactivation of factor VIII: a crucial role for proteolytic cleavage at Arg336 responsible for plasmin‐catalyzed factor VIII inactivation. J Biol Chem 2007; 282: 5287–95.
- 52Zeibdawi AR, Pryzdial EL. Mechanism of factor Va inactivation by plasmin. Loss of A2 and A3 domains from a Ca2+‐dependent complex of fragments bound to phospholipid. J Biol Chem 2001; 276: 19929–36.
- 53Lee CD, Mann KG. Activation/inactivation of human factor V by plasmin. Blood 1989; 73: 185–90.
- 54Hur WS, Mazinani N, Lu XJ, Britton HM, Byrnes JR, Wolberg AS, Kastrup CJ. Coagulation factor XIIIa is inactivated by plasmin. Blood 2015; 126: 2329–37.
- 55Niego BE, Medcalf RL. Plasmin‐dependent modulation of the blood‐brain barrier: a major consideration during tPA‐induced thrombolysis? J Cereb Blood Flow Metab 2014; 34: 1283–96.





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 