
相關資料:
1.專屬網站
2.諾華Novatis網站
DESCRIPTION
Xolair (Omalizumab) is a recombinant DNA-derived humanized IgG1ë monoclonal antibody that selectively binds to human immunoglobulin E (IgE). The antibody has a molecular weight of approximately 149 kilodaltons. Xolair is produced by a Chinese hamster ovary cell suspension culture in a nutrient medium containing the antibiotic gentamicin.
Gentamicin is not detectable in the final product.
Xolair is a sterile, white, preservative-free, lyophilized powder contained in a single-use vial that is reconstituted with Sterile Water for Injection (SWFI), USP, and administered as a subcutaneous (SC) injection. A Xolair vial contains
202.5 mg of Omalizumab, 145.5 mg sucrose, 2.8 mg L-histidine hydrochloride monohydrate, 1.8 mg L-histidine,and 0.5 mg polysorbate 20, and is designed to deliver 150 mg of Omalizumab in 1.2 mL after reconstitution with 1.4 mL SWFI, USP.
CLINICAL PHARMACOLOGY
Mechanism of Action
Xolair inhibits the binding of IgE to the high-affinity IgE receptor (FcåRI) on the surface of mast cells and basophils.
Reduction in surface-bound IgE on FcåRI-bearing cells limits the degree of release of mediators of the allergic response. Treatment with Xolair also reduces the number of FcåRI receptors on basophils in atopic patients.
Pharmacokinetics
After SC administration, Omalizumab is absorbed with an average absolute bioavailability of 62%. Following a single SC dose in adult and adolescent patients with asthma, Omalizumab was absorbed slowly, reaching peak serum
concentrations after an average of 7-8 days. The pharmacokinetics of Omalizumab are linear at doses greater than 0.5 mg/kg. Following multiple doses of Omalizumab, areas under the serum concentration-time curve from Day 0 to Day 14 at steady state were up to 6-fold of those after the first dose. In vitro, Omalizumab forms complexes of limited size with IgE. Precipitating complexes and complexes larger than
1 million daltons in molecular weight are not observed in vitro or in vivo. Tissue distribution studies in cynomolgus monkeys showed no specific uptake of 125I-Omalizumab by any organ or tissue. The apparent volume of distribution
in patients following SC administration was 78 ± 32 mL/kg.
Clearance of Omalizumab involves IgG clearance processes as well as clearance via specific binding and complex formation with its target ligand, IgE. Liver elimination of IgG includes degradation in the liver reticuloendothelial system (RES) and endothelial cells. Intact IgG is also excreted in bile. In studies with mice and monkeys,
Omalizumab:IgE complexes were eliminated by interactions with Fcç receptors within the RES at rates that were generally faster than IgG clearance. In asthma patients Omalizumab serum elimination half-life averaged 26 days, with apparent clearance averaging 2.4 ± 1.1 mL/kg/day. In addition, doubling body weight approximately doubled apparent clearance.
Pharmacodynamics
In clinical studies, serum free IgE levels were reduced in a dose dependent manner within 1 hour following the first dose and maintained between doses. Mean serum free IgE decrease was greater than 96% using recommended doses. Serum total IgE levels (i.e., bound and unbound) increased after the first dose due to the formation of
Omalizumab:IgE complexes, which have a slower elimination rate compared with free IgE. At 16 weeks after the first dose, average serum total IgE levels were five-fold higher compared with pre-treatment when using standard assays. After discontinuation of Xolair dosing, the Xolair-induced increase in total IgE and decrease in free IgE were reversible, with no observed rebound in IgE levels after drug washout. Total IgE levels did not return to pretreatment levels for up to one year after discontinuation of Xolair.
Special Populations
The population pharmacokinetics of Xolair were analyzed to evaluate the effects of demographic characteristics.
Analyses of these limited data suggest that no dose adjustments are necessary for age (12-76 years), race, ethnicity,or gender.
INDICATIONS AND USAGE
Xolair (Omalizumab) is indicated for adults and adolescents (12 years of age and above) with moderate to severe persistent asthma who have a positive skin test or in vitro reactivity to a perennial aeroallergen and whose symptoms are inadequately controlled with inhaled corticosteroids. Xolair has been shown to decrease the incidence of asthma exacerbations in these patients. Safety and efficacy have not been established in other allergic conditions.
CONTRAINDICATIONS
Xolair should not be administered to patients who have experienced a severe hypersensitivity reaction to Xolair (see WARNINGS: Anaphylaxis).
WARNINGS
Malignancy
Malignant neoplasms were observed in 20 of 4127 (0.5%) Xolair-treated patients compared with 5 of 2236 (0.2%)control patients in clinical studies of asthma and other allergic disorders. The observed malignancies in Xolairtreated
patients were a variety of types, with breast, non-melanoma skin, prostate, melanoma, and parotid occurring more than once, and five other types occurring once each. The majority of patients were observed for less than 1 year. The impact of longer exposure to Xolair or use in patients at higher risk for malignancy (e.g., elderly, current smokers) is not known (see ADVERSE REACTIONS: Malignancy).
Anaphylaxis
Anaphylaxis has occurred within 2 hours of the first or subsequent administration of Xolair in 3 (< 0.1%) patients without other identifiable allergic triggers. These events included urticaria and throat and/or tongue edema (see ADVERSE REACTIONS). Patients should be observed after injection of Xolair, and medications for the treatment of
severe hypersensitivity reactions including anaphylaxis should be available. If a severe hypersensitivity reaction to Xolair occurs, therapy should be discontinued (see CONTRAINDICATIONS).
PRECAUTIONS
General
Xolair has not been shown to alleviate asthma exacerbations acutely and should not be used for the treatment of acute bronchospasm or status asthmaticus.
Corticosteroid Reduction Systemic or inhaled corticosteroids should not be abruptly discontinued upon initiation of Xolair therapy. Decreases
in corticosteroids should be performed under the direct supervision of a physician and may need to be performed gradually.
Information for Patients
Patients receiving Xolair should be told not to decrease the dose of, or stop taking any other asthma medications
unless otherwise instructed by their physician. Patients should be told that they may not see immediate improvement
in their asthma after beginning Xolair therapy.
Parasitic (Helminth) Infection
In a one-year clinical trial conducted in Brazil in patients at high risk for geohelminthic infections (roundworm, hookworm,
whipworm, threadworm), 53% (36/68) of Omalizumab-treated patients experienced an infection, as diagnosed
by standard stool examination, compared to 42% (29/69) of placebo controls. The point estimate of the odds
ratio for infection was 1.96, with a 95% confidence interval (0.88, 4.36) indicating that in this study a patient who
had an infection was anywhere from 0.88 to 4.36 times as likely to have received Omalizumab than a patient who did
not have an infection. Response to appropriate anti-geohelminth treatment of infection as measured by stool egg
counts was not different between treatment groups. Patients at high risk of geohelminth infection should be monitored
for such infections while on Xolair therapy. Insufficient data are available to determine the length of monitoring
required for geohelminth infections after stopping Xolair treatment.
Drug Interactions
No formal drug interaction studies have been performed with Xolair. The concomitant use of Xolair and allergen
immunotherapy has not been evaluated.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term studies have been performed in animals to evaluate the carcinogenic potential of Xolair.
No evidence of mutagenic activity was observed in Ames tests using six different strains of bacteria with and without
metabolic activation at Omalizumab concentrations up to 5000 μg/mL.
The effects of Omalizumab on male and female fertility have been assessed in cynomolgus monkey studies. Administration
of Omalizumab at doses up to and including 75 mg/kg/week did not elicit reproductive toxicity in male
cynomolgus monkeys and did not inhibit reproductive capability, including implantation, in female cynomolgus
monkeys. These doses provide a 2- to 16-fold safety factor based on total dose and 2- to 5-fold safety factor based
on AUC over the range of adult clinical doses.
Pregnancy (Category B)
Reproduction studies in cynomolgus monkeys have been conducted with Omalizumab. Subcutaneous doses up to
75 mg/kg (12-fold the maximum clinical dose) of Omalizumab did not elicit maternal toxicity, embryotoxicity, or
teratogenicity when administered throughout organogenesis and did not elicit adverse effects on fetal or neonatal
growth when administered throughout late gestation, delivery, and nursing.
IgG molecules are known to cross the placental barrier. There are no adequate and well-controlled studies of Xolair
in pregnant women. Because animal reproduction studies are not always predictive of human response, Xolair
should be used during pregnancy only if clearly needed.
Nursing Mothers
The excretion of Omalizumab in milk was evaluated in female cynomolgus monkeys receiving SC doses of
75 mg/kg/week. Neonatal plasma levels of Omalizumab after in utero exposure and 28 days of nursing were between
11% and 94% of the maternal plasma level. Milk levels of Omalizumab were 1.5% of maternal blood concentration.
While Xolair presence in human milk has not been studied, IgG is excreted in human milk and therefore it is expected
that Xolair will be present in human milk. The potential for Xolair absorption or harm to the infant are unknown; caution
should be exercised when administering Xolair to a nursing woman.
Pediatric Use
Safety and effectiveness in pediatric patients below the age of 12 have not been established.
Geriatric Use
In clinical trials 134 patients 65 years of age or older were treated with Xolair. Although there were no apparent agerelated
differences observed in these studies, the number of patients aged 65 and over is not sufficient to determine
whether they respond differently from younger patients.
ADVERSE REACTIONS
The most serious adverse reactions occurring in clinical studies with Xolair are malignancies and anaphylaxis (see
WARNINGS). The observed incidence of malignancy among Xolair-treated patients (0.5%) was numerically higher
than among patients in control groups (0.2%). Anaphylactic reactions were rare but temporally associated with
Xolair administration.
The adverse reactions most commonly observed among patients treated with Xolair included injection site reaction
(45%), viral infections (23%), upper respiratory tract infection (20%), sinusitis (16%), headache (15%), and pharyngitis
(11%). These events were observed at similar rates in Xolair-treated patients and control patients. These were
also the most frequently reported adverse reactions resulting in clinical intervention (e.g., discontinuation of Xolair,
or the need for concomitant medication to treat an adverse reaction).
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical
trials of one drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the
rates observed in medical practice.
The data described above reflect Xolair exposure for 2076 adult and adolescent patients ages 12 and older, including
1687 patients exposed for six months and 555 exposed for one year or more, in either placebo-controlled or other
controlled asthma studies. The mean age of patients receiving Xolair was 42 years, with 134 patients 65 years of age
or older; 60% were women, and 85% Caucasian. Patients received Xolair 150 to 375 mg every 2 or 4 weeks or, for
patients assigned to control groups, standard therapy with or without a placebo.
Table 4 shows adverse events that occurred ³ 1% more frequently in patients receiving Xolair than in those receiving
placebo in the placebo-controlled asthma studies. Adverse events were classified using preferred terms from the
International Medical Nomenclature (IMN) dictionary.
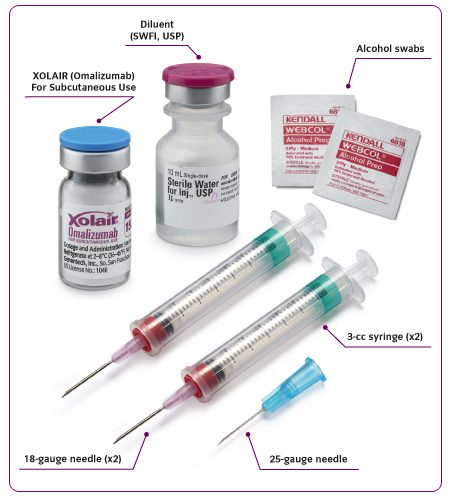
Preparation for Administration
Xolair for SC administration should be prepared using SWFI, USP, ONLY.
Xolair is for single use only and contains no preservatives. The solution should be used for SC administration within
8 hours following reconstitution when stored in the vial at 2-8°C (36-46°F), or within 4 hours of reconstitution when
stored at room temperature.
The lyophilized product takes 15-20 minutes to dissolve. The fully reconstituted product will appear clear or slightly
opalescent and may have a few small bubbles or foam around the edge of the vial. The reconstituted product is
somewhat viscous; in order to obtain the full 1.2 mL dose, ALL OF THE PRODUCT MUST BE WITHDRAWN from the
vial before expelling any air or excess solution from the syringe.
STEP 1: Draw 1.4 mL of SWFI, USP into a 3-cc syringe equipped with a 1-inch, 18-gauge needle.
STEP 2: Place the vial upright on a flat surface and using standard aseptic technique, insert the needle and inject the
SWFI, USP directly onto the product.
STEP 3: Keeping the vial upright, gently swirl the upright vial for approximately 1 minute to evenly wet the powder.
Do not shake.
STEP 4: After completing STEP 3, gently swirl the vial for 5-10 seconds approximately every 5 minutes in order to
dissolve any remaining solids. There should be no visible gel-like particles in the solution. Do not use if foreign particles
are present.
Note: Some vials may take longer than 20 minutes to dissolve completely. If this is the case, repeat STEP 4 until
there are no visible gel-like particles in the solution. It is acceptable to have small bubbles or foam around the edge
of the vial. Do not use if the contents of the vial do not dissolve completely by 40 minutes.
STEP 5: Invert the vial for 15 seconds in order to allow the solution to drain toward the stopper. Using a new 3-cc
syringe equipped with a 1-inch, 18-gauge needle, insert the needle into the inverted vial. Position the needle tip at the
very bottom of the solution in the vial stopper when drawing the solution into the syringe. Before removing the needle
from the vial, pull the plunger all the way back to the end of the syringe barrel in order to remove all of the solution
from the inverted vial.
STEP 6: Replace the 18-gauge needle with a 25-gauge needle for subcutaneous injection.
STEP 7: Expel air, large bubbles, and any excess solution in order to obtain the required 1.2 mL dose. A thin layer of
small bubbles may remain at the top of the solution in the syringe. Because the solution is slightly viscous, the injection
may take 5-10 seconds to administer.
A vial delivers 1.2 mL (150 mg) of Xolair. For a 75 mg dose, draw up 0.6 mL into the syringe and discard the remaining
product
Stability and Storage
Xolair should be shipped at controlled ambient temperature (² 30°C [² 86°F]). Xolair should be stored under refrigerated conditions 2-8°C (36-46°F). Do not use beyond the expiration date stamped on carton.
Xolair is for single-use only and contains no preservatives. The solution may be used for SC administration within 8 hours following reconstitution when stored in the vial at 2-8°C (36-46°F), or within 4 hours of reconstitution when
stored at room temperature.
Reconstituted Xolair vials should be protected from direct sunlight.
抗IgE藥物,目前也有部分是用在川崎氏症的患者身上。





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 