這幾天有篇醫學報導強力攻佔了各媒體的版面,摘錄報導如下(並非針對聯合新聞,中央社、東森、中廣、路透...等等也有同樣的新聞)
而事實上,原文如下:
Results
Data were combined by means of a fixed-effects model. In the 42 trials, the mean age of the subjects was approximately 56 years, and the mean baseline glycated hemoglobin level was approximately 8.2%. In the rosiglitazone group, as compared with the control group, the odds ratio for myocardial infarction was 1.43 (95% confidence interval [CI], 1.03 to 1.98; P = 0.03), and the odds ratio for death from cardiovascular causes was 1.64 (95% CI, 0.98 to 2.74; P = 0.06).
Conclusions
Rosiglitazone was associated with a significant increase in the risk of myocardial infarction and with an increase in the risk of death from cardiovascular causes that had borderline significance. Our study was limited by a lack of access to original source data, which would have enabled time-to-event analysis. Despite these limitations, patients and providers should consider the potential for serious adverse cardiovascular effects of treatment with rosiglitazone for type 2 diabetes.
Discussion
Our data show that, as compared with placebo or with other antidiabetic regimens, treatment with
rosiglitazone was associated with a significant increase in the risk of myocardial infarction and with an increase in the risk of death from cardiovascular causes that was of borderline significance.
The similar odds ratio for comparison with placebo suggests that the increased risk associated with rosiglitazone was not a function of the protective effects of active comparator drugs. However, these findings are based on limited access to trial results from publicly available sources, not on patient-level source data. Furthermore, results are based on a relatively small number of events, resulting in odds ratios that could be affected by small changes in the classification of events. Nonetheless, our findings are worrisome because of the high incidence of cardiovascular events in patients with diabetes.4 Because exposure of such patients to rosiglitazone is widespread, the public health impact of an increase in cardiovascular risk could be substantial if our data are borne out by further analysis and the results of larger controlled trials.
由此可知,作者並未肯定rosiglitazone對於致死性心肌梗塞的直接關係,而且他也親自說明,所採用的証據只是由先前其他40幾項研究中所重新統計而計算出來的,況且受侍者人數也仍為少數,,因此整體資料並不完整,所使用的研究方法「連作者都承認明顯受限」。只能說對此類藥物的使用上必須更加小心,而非呼籲大眾此類藥物的致死性。
此外,附上一張原文的表格
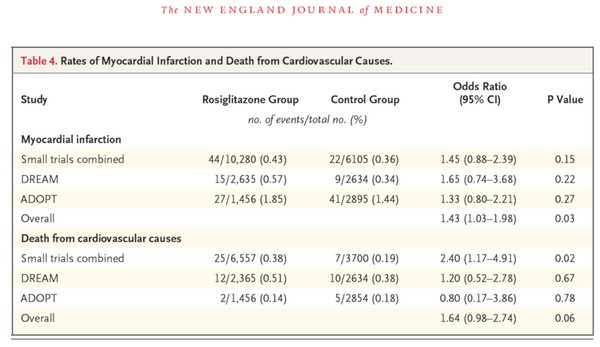
由表格中可以明顯得知,數據在統計學上並沒有顯著的差異性,所以結論跟作者所說相似,都是需要更進一步的研究及觀察。
也因此,FDA審視糖尿病藥物部門的負責人Dr. Robert Meyer說,其它研究的數據與該項研究結果相悖。 FDA尚未確認其他研究報告中所稱風險增加的臨床含義,目前不會要求葛蘭素史克美占採取任何行動,但會要求公共諮詢委員會在未來進行評估。
而針對此類藥物,Thiazolidinedione的藥物由於機轉因素,本身就會有鈉滯留及加重水腫的副作用,這些在仿單上面以明確記載,而在藥典之中,也有明顯列出副作用並警告凡有心衰竭、肺水腫、骨折、肝膽換及的病患應小心甚至避免使用。此次事件最重要的,是在提醒所有的醫事人員,都應該確實瞭解病患的病使及病情,小心的為病患選擇合適的用藥,以及有任何藥物上面的問題,一定要跟藥師諮詢。而醫師、藥師、護士讓患者服用這種藥物時,也要定期檢測患者的肝功能指數,同時也呼籲糖尿病患者,不要擅自停藥,如果有心臟病史,應該諮詢醫師及藥師,評估是否更換藥物。切勿自行停藥,以免加重疾病的惡化。
而針對媒體來說,許多媒體為了增加版面的曝光率而以聳動斗大的標題,大肆渲染嚴重的副作用,然而事實並非是這樣,這種煽動民眾的行為實在不可取,而所有的民眾也應該在有類似新聞時,去請問自己的醫師或是藥師事件的真實性。同樣的道理,所有的醫事人員在看到相關報導時,更應該拿出自己所受過的專業訓練,徹底瞭解文章的內容,並審慎的評估文章的內容,才不會誤導民眾做出不正確的用藥行為。。
服「梵帝雅」糖尿病藥 心臟病發增43%
卓具聲望的「新英格蘭醫學期刊」廿一日發表一項分析報告指出,使用普遍的糖尿病藥物梵帝雅(Avandia)會使心臟病發作的機率提高百分之四十三,死於心臟病的風險也會增加。
美國食品藥物管理局(FDA)已對此藥發出「安全警告」,建議服用此藥的病人,特別是屬心臟病發作高危險群者,尋求醫生指示。
此事已使食品藥物管理局遭受監督不周的批評。該局負責糖尿病藥物審核的梅爾博士表示,一些其他研究的結論與前述發現並不相符,該局尚未確認這些增加的風險在臨床上的意義,目前該局並未要求生產廠商葛蘭素史克公司採取任何行動。
葛蘭素史克公司也發表聲明,表示極不同意這篇報告的結論,指其證據不完整,分析方法也有缺陷。該公司堅決支持正確服用梵帝雅的安全,相信其重大藥效超越任何治療的風險。
新英格蘭醫學期刊將在六月十四日出版的印刷期刊中刊出此一分析報告,但在考慮對公共衛生的重要性後,決定於廿一日在其網站先行公布,由於公布時間意外選在下午五時之前,以致生產該藥的英國葛蘭素史克製藥公司股票在華爾街股市下大跌,收盤價下挫百分之七點九,在倫敦也跌了百分之五。
該期刊的分析報告指出,在對四十四項有關此藥的研究進行分析後,發現與服用他種糖尿病藥物及未服糖尿病藥者相比,該藥使心臟病發作的危險增加百分之四十三。這項分析是由克利夫蘭診所的心臟血管醫學主任尼森和另一名醫生凱希‧伍斯基所做的。尼森也是最早質疑默克藥廠現在已撤回的止痛藥偉克適有害心臟醫生之一,他在去年十二月投書刺胳針(Lancet)醫學期刊,提出對梵帝雅的懷疑。
而事實上,原文如下:
Results
Data were combined by means of a fixed-effects model. In the 42 trials, the mean age of the subjects was approximately 56 years, and the mean baseline glycated hemoglobin level was approximately 8.2%. In the rosiglitazone group, as compared with the control group, the odds ratio for myocardial infarction was 1.43 (95% confidence interval [CI], 1.03 to 1.98; P = 0.03), and the odds ratio for death from cardiovascular causes was 1.64 (95% CI, 0.98 to 2.74; P = 0.06).
Conclusions
Rosiglitazone was associated with a significant increase in the risk of myocardial infarction and with an increase in the risk of death from cardiovascular causes that had borderline significance. Our study was limited by a lack of access to original source data, which would have enabled time-to-event analysis. Despite these limitations, patients and providers should consider the potential for serious adverse cardiovascular effects of treatment with rosiglitazone for type 2 diabetes.
Discussion
Our data show that, as compared with placebo or with other antidiabetic regimens, treatment with
rosiglitazone was associated with a significant increase in the risk of myocardial infarction and with an increase in the risk of death from cardiovascular causes that was of borderline significance.
The similar odds ratio for comparison with placebo suggests that the increased risk associated with rosiglitazone was not a function of the protective effects of active comparator drugs. However, these findings are based on limited access to trial results from publicly available sources, not on patient-level source data. Furthermore, results are based on a relatively small number of events, resulting in odds ratios that could be affected by small changes in the classification of events. Nonetheless, our findings are worrisome because of the high incidence of cardiovascular events in patients with diabetes.4 Because exposure of such patients to rosiglitazone is widespread, the public health impact of an increase in cardiovascular risk could be substantial if our data are borne out by further analysis and the results of larger controlled trials.
由此可知,作者並未肯定rosiglitazone對於致死性心肌梗塞的直接關係,而且他也親自說明,所採用的証據只是由先前其他40幾項研究中所重新統計而計算出來的,況且受侍者人數也仍為少數,,因此整體資料並不完整,所使用的研究方法「連作者都承認明顯受限」。只能說對此類藥物的使用上必須更加小心,而非呼籲大眾此類藥物的致死性。
此外,附上一張原文的表格

由表格中可以明顯得知,數據在統計學上並沒有顯著的差異性,所以結論跟作者所說相似,都是需要更進一步的研究及觀察。
也因此,FDA審視糖尿病藥物部門的負責人Dr. Robert Meyer說,其它研究的數據與該項研究結果相悖。 FDA尚未確認其他研究報告中所稱風險增加的臨床含義,目前不會要求葛蘭素史克美占採取任何行動,但會要求公共諮詢委員會在未來進行評估。
而針對此類藥物,Thiazolidinedione的藥物由於機轉因素,本身就會有鈉滯留及加重水腫的副作用,這些在仿單上面以明確記載,而在藥典之中,也有明顯列出副作用並警告凡有心衰竭、肺水腫、骨折、肝膽換及的病患應小心甚至避免使用。此次事件最重要的,是在提醒所有的醫事人員,都應該確實瞭解病患的病使及病情,小心的為病患選擇合適的用藥,以及有任何藥物上面的問題,一定要跟藥師諮詢。而醫師、藥師、護士讓患者服用這種藥物時,也要定期檢測患者的肝功能指數,同時也呼籲糖尿病患者,不要擅自停藥,如果有心臟病史,應該諮詢醫師及藥師,評估是否更換藥物。切勿自行停藥,以免加重疾病的惡化。
而針對媒體來說,許多媒體為了增加版面的曝光率而以聳動斗大的標題,大肆渲染嚴重的副作用,然而事實並非是這樣,這種煽動民眾的行為實在不可取,而所有的民眾也應該在有類似新聞時,去請問自己的醫師或是藥師事件的真實性。同樣的道理,所有的醫事人員在看到相關報導時,更應該拿出自己所受過的專業訓練,徹底瞭解文章的內容,並審慎的評估文章的內容,才不會誤導民眾做出不正確的用藥行為。。
全站熱搜





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 