今天來練習一下抗潰瘍藥物吧
先來個前測:
1.Which of these proton-pump inhibitors are not affected by food intake?
A.Esomeprazole and omeprazole
B.Pantoprazole and rabeprazole
C.Dexlansoprazole and pantoprazole
D.Dexlansoprazole and lansoprazole
2.On the basis of data after 8 weeks of FDA-approved doses, which of these proton-pump inhibitors has been shown to be superior to omeprazole for the healing of erosive esophagitis?
A.Esomeprazole
B.Immediate-release omeprazole
C.Dexlansoprazole
D.Pantoprazole
選好了嗎?接下來先上個課
Introduction
Proton-pump inhibitors (PPIs) are among the most commonly used medications in the world today.[1] Since the introduction in the late 1980s of the first commercially available PPI, omeprazole, this class of medications has revolutionized the way in which clinicians manage acid-related disorders of the gastrointestinal (GI) tract; these agents have become the standard of care for the treatment of gastroesophageal reflux disease (GERD), peptic ulcer disease (PUD), and dyspepsia, the most commonly encountered conditions resulting in upper GI complaints. Even though examination of the approved uses of the various PPIs reveals that the specific indications related to the treatment of GERD and PUD differ slightly among the PPIs and that none of these agents is explicitly indicated for the treatment of dyspepsia, most clinicians consider them largely interchangeable in their ability to relieve upper GI symptoms thought to be acid mediated.[2-8] Six different PPIs are currently approved by the US Food and Drug Administration (FDA): omeprazole, esomeprazole, lansoprazole, dexlansoprazole, pantoprazole, and rabeprazole. These agents are available in a variety of different formulations and administration forms. The PPIs are all remarkably similar in regard to how they work to decrease gastric acid secretion, and yet there are important differences in these medications that can, along with differences in individual patient genetics and physiology, influence the success or failure of these medications to control acid-related GI symptoms. Knowledge of the pharmacokinetic and pharmacodynamic differences among the various PPIs is important for the clinician who treats patients with GERD, PUD, and dyspepsia. This article reviews the physiology of gastric acid secretion and the mechanism of action and potentially clinically relevant differences among the PPIs that are currently available for use.
Physiology of Gastric Acid Secretion
Gastric parietal cells are the acid-producing cells of the stomach. Parietal cells contain a region known as the secretory canaliculus; it is the most acidic environment (pH ≈ 0.8) in the human body and is the cellular site where acid is secreted into the gastric lumen. Gastric acid secretion into the gastric lumen occurs in response to messages received via a variety of hormonal, paracrine, and neurocrine inputs. Gastrin, produced by G cells located in the pyloric mucosa of the stomach, is the primary hormonal stimulation for gastric acid production.[9]Paracrine and neurocrine messengers that stimulate gastric acid secretion include histamine and acetylcholine, released from enterochromaffin-like (ECL) cells and neuronal synapses, respectively. Natural inhibitors of gastric acid secretion include the paracrine release of somatostatin from gastric D cells and the hormones cholecystokinin (CCK), secretin, neurotensin, and glucagon-like peptide (GLP).[9] The various inputs that stimulate parietal cells to secrete hydrogen ions into the gastric lumen have different levels of functional importance, but it is the histamine-2 receptors that are thought to be the primary stimulus for acid secretion. When gastrin is released from G cells in response to a meal, it binds to CCK-2 receptors on both the parietal and ECL cells. The binding of gastrin to the parietal cell results in release of intracellular calcium and translocation and activation of the H+, K+-ATPase (the proton pump).[9] Gastrin binding to the ECL cell promotes the formation and creation of histamine which then binds to H2 receptors on the parietal cell to increase production of cyclic AMP (cAMP), which is followed by translocation and activation of the proton pump at the canalicular membrane. In a healthy body, all of these prosecretory steps are accompanied by corresponding stimulation of antisecretory mechanisms that counter the equation to maintain the intragastric pH at an average of 1.4 (Figure 1).[9]

Figure 1. Hormonal regulation of gastric acid secretion. From Schubert ML.[9]
The gastric H+, K+-ATPase is one of a family of ATPase enzymes for which cycles of phosphorylation and dephosphorylation result in conformational changes of the enzyme spanning the cytoplasmic membrane. These changes facilitate ion transport between the intracellular and extracellular environments of the cell. The conformations of the H+, K+-ATPase that permit H+ion transport out of the cell are termed E1, whereas those that permit inward K+ transport are termed E2 conformations.[10] These conformations are mutually exclusive so that ion transport can only occur in a unidirectional fashion via the proton pump at a given point in time.
PPI Pharmacology
All of the PPIs are weak bases that accumulate in the acidic environment of the secretory canaliculus, where they are transformed into thiophilic intermediates that bind to cysteine sulfhydryl groups on the luminal aspect of the proton pump to form covalent disulfide bonds.[11] The formation of these disulfide bonds locks the H+,K+-ATPase into the E2 conformation, thus preventing additional ion exchange and H+ secretion into the gastric lumen. The degree of PPI accumulation in the secretory canaliculus and rate of conversion to the active sulfenamide form of the drug are the rate-limiting steps in determining the onset of acid inhibition of a PPI.[11] These steps are dependent on the pKa of each PPI, which is variable. For example, the pKa of rabeprazole (4.53) is the highest among the available PPIs and is therefore the first to be activated, whereas dexlansoprazole, lansoprazole, and pantoprazole all have a pKa of 3.83 and are activated at a slower rate than rabeprazole.[11]
All of the currently available PPIs are derived from a precursor molecule developed in the 1970s, known as timoprazole.[12] PPIs available in the United States are all modeled on this molecule and consist of 2 ring portions: a pyridine ring and a benzimidazole (Figure 2). Substitution on these rings confers differential activation properties to each PPI. Once activated, all of the PPIs bind to cysteine 813 in the proton pump and some PPIs bind to cysteine 822 as well as other anionic residues, such as glutamine 820.[11] All PPIs are acid labile and require protection from gastric acid to prevent premature activation and binding to cysteine moieties of intragastric proteins before they are absorbed into the systemic circulation and redistributed to the secretory canaliculus.[13] Most of the currently available conventional PPIs are delayed-release preparations whereby the active drug is protected from premature gastric acid activation by an enteric coating. Dexlansoprazole, the newest PPI to be introduced to the market, has a dual-delayed-release formulation using 2 different methacrylic acid copolymer-coated PPI granules with slightly different pH-dependent releases packaged within the same capsule.[14] These different enteric coatings are designed to provide extended exposure of the proton pumps to dexlansoprazole, theoretically providing a greater degree of gastric acid secretion compared with other PPIs with plasma half-lives of 1.5-2 hours. With this formulation, approximately 25% of the dexlansoprazole is released in a manner consistent with other PPIs, in the proximal small bowel, resulting in a first peak of plasma levels 1-2 hours after ingestion. The other 75% of the drug releases distally in the small bowel, resulting in a second plasma peak 4-5 hours after ingestion, theoretically prolonging the exposure of actively secreting proton pumps to circulating PPI. A comparison of both the pharmacokinetic and pharmacodynamic effects of dexlansoprazole with this enteric-coating scheme vs conventional lansoprazole did show that the dual-delayed-release formulation resulted in significantly higher postdose plasma concentrations and delayed clearance.[15] Clinically, this translated to significantly higher intragastric pH when patients took dexlansoprazole vs lansoprazole.
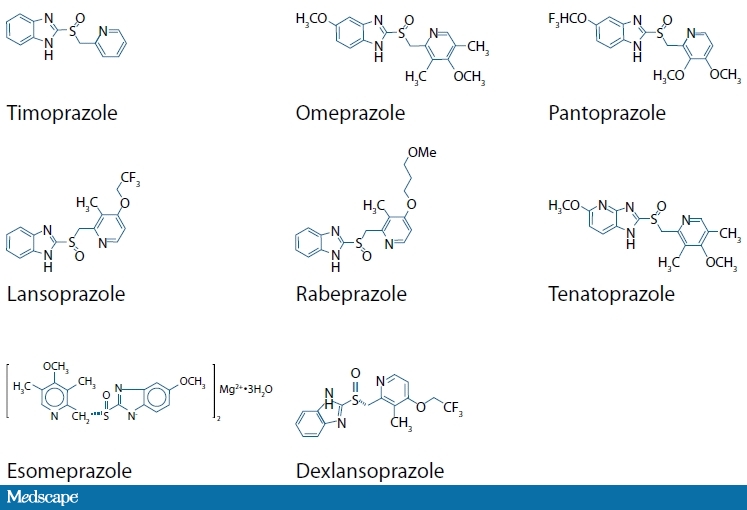
Figure 2. Molecular structure of various PPIs.[3,5,16] (The structure for immediate-release omeprazole is omeprazole without the enteric coating packaged with bicarbonate.)
The only non-delayed-release PPI formulation that is currently marketed is immediate-release omeprazole (IR-OME). This formulation consists of omeprazole protected from intragastric acid activation by sodium bicarbonate.[17] The lack of enteric coating of IR-OME and the fact that it is administered as a liquid gives this preparation a theoretical advantage over other PPIs in regard to the onset of acid suppression and perhaps reduction in nocturnal acid breakthrough, although no convincing studies to date have shown this agent to be clinically superior to other PPIs for commonly used indications (GERD, PUD, or dyspepsia).
Once PPIs are absorbed from the small intestine, they are redistributed via the systemic circulation and accumulate in the acidic environment of the stomach where they undergo activation. They are then able to bind to and block actively secreting proton pumps. There is individual patient variability in gastric acid inhibition in response to PPIs; this is based on multiple factors, such as intestinal absorption, cytochrome P450 (CYP) metabolism, and genetic polymorphisms of the proton pump itself, which make predicting individual dose response difficult.[18] It is recommended that PPIs be administered before meals because this is the time when more proton pumps are actively secreting acid into the gastric lumen. The digestive period creates the proper environment for PPI activation and also corresponds to the scenario in which the H+, K+-ATPase is more likely to be in the proper E1 conformation to permit PPI-mediated covalent inhibition. Pantoprazole and dexlansoprazole, however, do not appear to be affected by food intake and can be taken without regard to meals or the timing of meals in most patients.[19,20]
Recovery of gastric acid secretion after PPI inhibition is thought to occur by 2 mechanisms. The best established of these relies on the formation of new H+, K+-ATPase units and their intracellular transport from intracytoplasmic tubules to the canalicular membrane.[21] Although the inhibition achieved with PPIs was previously thought to be irreversible, the fact that acid secretion returns sooner than should occur if recovery were totally dependent on new pump formation has led to the supposition that the bonds that form between the activated PPI and the cysteine residues in the H+, K+-ATPase can be reduced by agents such as glutathione. These hypotheses have been confirmed in animal studies.[21] This reversibility has been shown to be possible for all of the first-generation PPIs with the exception of pantoprazole. Acid recovery after administration of pantoprazole occurs at a rate that is more consistent with pump recovery only. One of the possible explanations for this is that pantoprazole also forms a disulfide bond with cysteine 822, which may be less accessible to reversal agents. Whether the more recently introduced PPIs (esomeprazole and dexlansoprazole) are subject to the reversal of disulfide bonds remains to be proven. One could imagine, however, that PPI resistance to such reversal could be clinically important and confer distinct advantages compared with PPIs that are subject to reversal.
PPI Metabolism
PPI metabolism has been a hot topic recently with the release of data suggesting that PPIs may interfere with the effects of clopidogrel, a platelet inhibitor commonly used to reduce the incidence of thrombotic cardiovascular events, and thus perhaps increase the incidence of such events in at-risk patients.[22] Clopidogrel, like the PPIs, requires conversion to an active form, although in the case of clopidogrel this activation occurs via specific P450 enzymes in the liver rather than via gastric acid.[23] It has been recognized that polymorphisms in the CYP2C19 alleles can result in altered responses to clopidogrel in patients harboring these mutations.[23,24] Thus, it is possible that other drugs, such as PPIs that are metabolized by the same P450 enzymes as clopidogrel, could affect the latter's activation and antiplatelet effects. Although all PPIs undergo extensive hepatic metabolism, there is variability among the different PPI formulations in terms of which P450 enzymes are involved as well as in the effects that some of the PPIs may have on the enzymes that are responsible for their metabolism.[11] Several recent studies have examined the possible effects of various PPIs on the platelet effects of clopidogrel and have produced provocative but variable results.[25-27]Whether the metabolism and action of prasugrel, a non-FDA-approved P2Y12 inhibitor (like clopidogrel), is affected by PPI coadministration remains unknown, but it is unlikely given that prasugrel is not subject to CYP2C19 activation.[28]
Omeprazole and esomeprazole are both metabolized by CYP2C19 and CYP3A4 of the P450 enzyme system. Compared with omeprazole, however, esomeprazole metabolism is more dependent on CYP3A4, which has a much slower elimination pathway. This preferentially slower metabolism of esomeprazole may be largely responsible for the superior pharmacodynamic and clinical results reported with esomeprazole compared with omeprazole.[29] Both of these PPIs also inhibit CYP2C19, and therefore their own metabolism, with chronic dosing. Recent data indicate that omeprazole may play a role in reducing the platelet effects of clopidogrel in patients undergoing coronary artery stenting who were subsequently randomly assigned to receive aspirin and clopidogrel along with either placebo or omeprazole 20 mg.[25] Compared with values on day 1, the platelet reactivity index (PRI) was significantly increased (indicating a greater propensity to clot) in patients who received omeprazole compared with those who received placebo. It should be emphasized, however, that the outcome of this study is not a clinical outcome. A large, nested, case-control study from Canada of patients with a history of myocardial infarction did suggest a link between PPI use and subsequent readmission for myocardial infarction in patients using clopidogrel.[26] Multiple logistic regression analysis of these data also indicated that this relationship was not present for pantoprazole. Another recent study, again looking at the effect of PPIs on PRI, found that neither pantoprazole nor esomeprazole appeared to have a significant effect on PRI.[27] In contrast to the Canadian study, however, a recently published study in The New England Journal of Medicine did not find that omeprazole (nor PPIs in general) was associated with an increased risk for coronary events in at-risk patients treated with clopidogrel.[30] Rather, these investigators corroborated the findings of other investigators that the risk for increased coronary events in patients taking clopidogrel appears to originate in polymorphisms of the CYP2C19 enzyme that are associated with loss of function, whereby less clopidogrel is activated in patients with these alleles.[23]Among the other PPIs, rabeprazole appears to have the lowest dependence on CYP metabolism, lansoprazole (like omeprazole and esomeprazole) is thought to also have some inhibitory effects on CYP2C19, and pantoprazole does not appear to inhibit this isoform.[11]
Because all of the PPIs reduce gastric acid, all of the package inserts/prescribing information for this class of medications recommend that care be used when coadministering PPIs and medications for which gastric pH is an important determinant of bioavailability, such as digoxin, ampicillin, iron salts, and ketoconazole.[2-8] Additionally, because there have been reports of alterations in international normalized ratio (INR) in patients taking PPIs and warfarin, closer monitoring may be merited in patients taking both medications.[2-8] The prescribing information for nearly all of the PPIs also recommends that PPIs not be used with certain antiretrovirals, such as atazanavir, because systemic concentrations of the antiretrovirals may be significantly reduced in the presence of PPIs.[2,3,5-8] Conversely, increased serum levels of medications such as saquinavir and tacrolimus have been reported with coadministration of some PPIs.[3] The significance and mechanisms of these altered drug levels is not completely understood. (The aforementioned possible interactions are not meant to be an all-inclusive list; the reader is encouraged to review the prescribing information and relevant literature regarding PPIs and possible drug interactions for individual patients.)
Do PPI Pharmacokinetics Affect Clinical Outcomes?
All of the PPIs are extremely effective acid inhibitors when administered at approved doses in nearly all patients with acid-mediated upper GI symptoms. Determining a rank order of PPI effectiveness is fraught with difficulty, based in part on artificial and unrealistic experimental design assumptions and the fact that biological systems differ in regard to the metabolism and activity of the different PPIs.[31] It is also important to keep in mind the different conditions that these agents are used to treat and to understand that the clinical and physiologic effects of PPIs may differ with respect to these conditions and the endpoints studied in individual trials. For example, one PPI may be as effective as another in an 8-week study with a primary endpoint of relief of heartburn symptoms in patients with nonerosive reflux disease, but it may not be as effective as another PPI in an 8-week study of healing erosive esophagitis. Many of the studies that have directly compared the various PPIs against each other use physiologic outcomes rather than clinical outcomes as the study endpoints. One comparison study of several delayed-release PPIs, dosed once daily in the morning, demonstrated equivalent control of intragastric pH during the daytime with universal return of some acid secretion during the overnight period,[32] whereas a 5-arm, open-label crossover study of delayed-release PPIs showed that esomeprazole 40 mg provided significantly better 24-hour intragastric pH control compared with omeprazole 20 mg, lansoprazole 30 mg, rabeprazole 20 mg, and pantoprazole 40 mg* in patients with GERD.[33] The clinical significance of studies such as these, however, is unclear.
The results of studies such as those cited above or others that have demonstrated physiologic or metabolic differences among the various PPIs raise the possibility that these pharmacokinetic differences can result in differential clinical results. The longer elimination half-lives of PPIs such as esomeprazole (due to differential CYP3A4 metabolism compared with omeprazole), dexlansoprazole (due to its dual-delayed-release formulation and second peak absorption profile), and an imidazopyridine-based PPI known as tenatoprazole* (with a longer elimination half-life) could theoretically result in more activated PPI being present in the parietal canaliculus available to inhibit previously unavailable proton pumps and thus result in better clinical outcomes. Some data support this, such as superior healing rates of erosive esophagitis observed with esomeprazole compared with omeprazole[34,35] and improved symptom resolution and healing rates with dexlansoprazole compared with lansoprazole in patients with erosive esophagitis.[36]
These observations notwithstanding, it has been reliably proven in the literature that the majority of patients with heartburn symptoms, endoscopy-proven esophagitis, and PUD benefit from any PPI administration.[37] Moreover, most data evaluating the first-generation delayed-release PPIs (omeprazole, lansoprazole, pantoprazole, and rabeprazole) point towards very similar clinical results accruing when equipotent doses of these medications are directly compared. Perhaps newer PPI formulations may be relegated to patients who demonstrate an incomplete response to first-generation PPIs, although this remains to be seen.
Conclusion
Although there are differences among the PPIs that may result in different clinical responses for individual patients who are prescribed these agents, the choice of which specific PPI to use will most likely be made on the basis of practitioner preference and experience as well as practical issues such as price, benefit coverage, and availability. For a small percentage of patients, careful consideration may be required to determine which PPI, and at what dose, will be most effective for a specific situation. Some of these patients may be partial responders to other PPIs or may have comorbidities or risks for drug interactions that may influence the choice of PPI to be used. Therefore, maintaining a working knowledge of the pharmacodynamic and pharmacokinetic properties and differences between the available PPIs is important for clinicians who will be prescribing these medications for patients with acid-mediated disorders.
This activity is supported by an independent educational grant from Takeda.
*The US Food and Drug Administration has not approved this medication/dosage for this use.
This article is a CME/CE certified activity. To earn credit for this activity visit:
http://cme.medscape.com/viewarticle/702505
References
- Cherry DK, Hing E, Woodwell DA, et al. National ambulatory medical care survey: 2006 summary. National Health Statistics Reports. 2008;3:1-40.
- PRILOSEC® (omeprazole). Prescribing information. Wilmington, Del: AstraZeneca Pharmaceuticals LP; 2008.
- NEXIUM® (esomeprazole magnesium). Prescribing information. Wilmington, Del: AstraZeneca Pharmaceuticals LP; 2008.
- PREVACID (lansoprazole). Prescribing information. Deerfield, Ill: Takeda Pharmaceuticals America, Inc; 2008.
- KAPIDEX™ (dexlansoprazole). Prescribing information. Deerfield, Ill: Takeda Pharmaceuticals America, Inc; 2009.
- PROTONIX® (pantoprazole). Prescribing information. Philadelphia, Pa: Wyeth Pharmaceuticals Inc; 2008.
- ACIPHEX® (rabeprazole sodium). Prescribing information. Manufactured and marketed by Eisai, Inc, Woodcliff Lake, NJ. Marketed by PRICARA, Division of Ortho-McNeil-Janssen Pharmaceuticals, Inc, Raritan, NJ; 2009.
- ZEGERID® (omeprazole/sodium bicarbonate). Prescribing information. San Diego, Calif: Santarus, Inc; 2008.
- Schubert ML. Hormonal regulation of gastric acid secretion. Curr Gastro Rep. 2008;10:523-527.
- Munson K, Garcia R, Sachs G. Inhibitor and ion binding sites on the gastric H,K-ATPase. Biochemistry. 2005;44:5267-5284.
- Roche VF. The chemically elegant proton pump inhibitors. Am J Pharm Educ. 2006;70:101.
- Shin JM, Cho YM, Sachs G. Chemistry of covalent inhibition of the gastric H+,K+-ATPase by proton pump inhibitors. J Am Chem Soc. 2004;126:7800-7811.
- Horn JR, Howden CW. Review article: similarities and differences among delayed-release proton-pump inhibitor formulations. Aliment Pharm Ther. 2005;22(Suppl 3):20-24.
- Metz DC, Vakily M, Dixit T, et al. Dual delayed release formulation of dexlansoprazole MR, a novel approach to overcome the limitations of conventional single release proton pump inhibitor therapy. Aliment Pharmacol Ther. 2009 Feb 27. [Epub ahead of print]
- Zhang W, Wu J, Atkinson S. Pharmacokinetic (PK), pharmacodynamic (PD) and safety evaluation of single and multiple 60 mg, 90 mg, and 120 mg oral doses of modified release Tak-390 (Tak-390mr) and 30 mg oral doses of lansoprazole (Lan) in healthy subjects. Gastroenterology. 2007;132:A487. [T1194]
- Shin JM, Cho YM, Sachs G. Chemistry of covalent inhibition of the gastric H+,K+-ATPase by proton pump inhibitors. J Am Chem Soc. 2004;126:7800-7811.
- Howden CW. Review article: immediate-release proton-pump inhibitor therapy -- potential advantages. Aliment Pharmacol Ther. 2005;22(Suppl 3):25-30.
- Katz P, Sachs G. Mechanism of action and safety of heartburn therapies. Pract Gastroenterol. 2003;27:80-88.
- Huber R, Hartmann M, Bliesath H, et al. Pharmacokinetics of pantoprazole in man. Int J Clin Pharmacol Ther. 1996;34:S7-S16.
- Lee RD, Vakily M, Mulford D, et al. Clinical trial: the effect and timing of food on the pharmacokinetics and pharmacodynamics of dexlansoprazole MR, a novel Dual Delayed Release formulation of a proton pump inhibitor--evidence for dosing flexibility. Aliment Pharmacol Ther. 2009;29:824-833.
- Shin JM, Sachs G. Restoration of acid secretion following treatment with proton pump inhibitors. Gastroenterology. 2002;123:1588-1597.
- PPI interactions with clopidogrel. Med Lett Drugs Ther. 2009;51:2-3.
- Mega JL, Close SL, Wiviott SD, et al. Cytochrome P-450 polymorphisms and response to clopidogrel. N Engl J Med. 2009;360:354-362.
- Freedman JE, Hylek EM. Clopidogrel, genetics, and drug responsiveness. N Engl J Med. 2009;360:411-413.
- Gilard M, Arnaud B, Cornily JC, et al. Influence of omeprazole on the antiplatelet action of clopidogrel associated with aspirin: the randomized, double-blind OCLA (Omeprazole CLopidogrel Aspirin) study. J Am Coll Cardiol. 2008;51:256-260.
- Juurlink DM, Gomes T, Ko DT, et al. A population-based study of the drug interaction between proton pump inhibitors and clopidogrel. CMAJ. 2009;180:713-718.
- Siller-Matula JM, Spiel AO, Lang IM, et al. Effects of pantoprazole and esomeprazole on platelet inhibition by clopidogrel. Am Heart J. 2009;157:148.e1-148.e5.
- Brandt JT, Close SL, Iturria SJ, et al. Common polymorphisms of CYP2C19 and CYP2C9 affect the pharmacokinetic and pharmacodynamic response to clopidogrel but not prasugrel. J Thromb Haemost. 2007;5:2429-2436.
- Andersson T. Single-isomer drugs: true therapeutic advances. Clin Pharmacokinet. 2004;43:279-285.
- Simon T, Verstuyft C, Mary-Krause M, et al. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med. 2009;360:363-375.
- Fock KM, Ang TL, Bee LC, et al. Proton pump inhibitors: Do differences in pharmacokinetics translate into differences in clinical outcomes? Clin Pharmacokinet. 2008;47:1-6.
- Tutuian R, Katz P, Castell D. A PPI is a PPI is a PPI: lessons from prolonged intragastric pH monitoring. Gastroenterology. 2000;118:A17. (Abstract)
- Miner P Jr, Katz PO, Chen Y, et al. Gastric acid control with esomeprazole, lansoprazole, omeprazole, pantoprazole, and rabeprazole: a five-way crossover study. Am J Gastroenterol. 2003;98:2616-2620.
- Kahrilas PJ, Falk GW, Johnson DA, et al. Esomeprazole improves healing and symptom resolution as compared with omeprazole in reflux oesophagitis patients: A randomized controlled trial. Aliment Pharmacol Ther. 2000;14:1249-1258.
- Richter JE, Kahrilas PJ, Johanson J, et al. Efficacy and safety of esomeprazole compared with omeprazole in GERD patients with erosive esophagitis: A randomized controlled trial. Am J Gastroenterol. 2001;96:656-665.
- Sharma P, Shaheen NJ, Perez MC, et al. Clinical trials: healing of erosive oesophagitis with dexlansoprazole MR, a proton pump inhibitor with a novel dual delayed-release formulation--results from two randomized controlled studies. Aliment Pharmacol Ther. 2009;29:731-741.
- Welage LS, Berardi RR. Evaluation of omeprazole, lansoprazole, pantoprazole, and rabeprazole in the treatment of acid-related diseases. J Am Pharm Assoc (Wash). 2000;40:52-62.
(本文作者:Brooks D. Cash, MD, FACP, FACG, AGAF)
好,教學就到這邊,如果對抗潰瘍藥還有興趣的,可以參考以下連結:
幽門螺旋桿菌的治療:http://mulicia.pixnet.net/blog/post/17894677
機轉圖:http://mulicia.pixnet.net/blog/post/22097539
接著,剛剛的答案呢?
1.Although optimal dosing of most proton-pump inhibitors (PPIs) is prior to mealtime, which PPIs are not affected by food intake?
Answer: Dexlansoprazole and pantoprazole
Pantoprazole and dexlansoprazole do not appear to be affected by food intake and can be taken without regard to meals or the timing of meals in most patients. It is important to recall that all PPIs require activation by exposure to gastric acid in order to block the H+, K+-ATPase. Although pantoprazole and dexlansoprazole are not exceptions to this rule, both have pharmacokinetic properties that allow them to have a longer exposure time to the gastric parietal cells and secretory canaliculus, which has been shown to not be significantly affected by food intake. For ease of instruction and intuitive optimization of PPI activation, however, it is recommended that PPIs be administered before meals because this is the time when more proton pumps are actively secreting acid into the gastric lumen.
2.Which of these PPIs has been shown to be superior to omeprazole for the healing of erosive esophagitis after 8 weeks of FDA-approved doses?
Answer: Esomeprazole
The longer elimination half-lives of PPIs such as esomeprazole (due to differential CYP3A4 metabolism compared with omeprazole), dexlansoprazole (due to its dual delayed-release formulation and second peak absorption profile), and an imidazopyridine-based PPI known as tenatoprazole* (with a longer elimination half-life) could theoretically result in more activated PPI being present in the parietal canaliculus available to inhibit previously unavailable proton pumps and thus result in better clinical outcomes. Some data support this, such as superior healing rates of erosive esophagitis observed with esomeprazole compared with omeprazole.
另外,還有一提是後測的,我就直接公布答案了
Which of these PPIs does not contain an enteric coating?
Answer: IR-OME
The only non-delayed-release PPI formulation that is currently marketed is IR-OME. This formulation consists of omeprazole protected from intragastric acid activation by sodium bicarbonate. The lack of an enteric coating on IR-OME and the fact that it is administered as a liquid gives this preparation a theoretical advantage over other PPIs in regard to the onset of acid suppression and possibly a reduction in nocturnal acid breakthrough, although no convincing studies to date have shown this agent to be clinically superior to other PPIs for commonly used indications (GERD, PUD, or dyspepsia).





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 