Abstract and Introduction
Introduction
Alzheimer's disease (AD) is characterized by loss of memory and intellectual function. It currently afflicts around 18 million people worldwide but the total numbers affected, if carers and relatives are included, are far greater. AD places enormous emotional and financial burdens on individuals and the state, and as more people are predicted to survive to old age, these burdens will increase if no effective treatment is discovered.
AD is categorized by age of onset (early versus late onset) and by whether there is an inherited component to the disease (familial versus sporadic). Except for a small number of earlyonset, familial AD cases that have a clear genetic component, the causes of AD are unclear, although risk factors for the disease are known and include increasing age, Down's syndrome, and possibly head injury.
Accumulating evidence suggests that infectious agents are important etiological factors in AD. Superficially, infectious agents such as viruses and bacteria might not seem likely candidates as causes of chronic diseases. This is perhaps because microbes are generally known to be the cause of many acute illnesses, and so they are assumed to vanish or to be expunged from the body when the illness ends. However, this reasoning fails to take into account the ability of many micro-organisms to remain in a dormant state until certain events reawaken them to a virulent state. This process of dormancy followed by activation makes infectious agents prime candidates as factors in chronic diseases. Certainly, there are a number of major precedents for the correctness of such a 'heretical' concept, for example, viruses in various cancers, and the bacterium Helicobacter pylori in stomach ulcers [Marshall and Warren, 1984].
In the case of AD, several agents have been proposed but the focus of this review is the evidence for an involvement of herpes simplex virus type 1 (HSV1). The rationale for implicating HSV1, a neurotropic virus, in AD is based on several facts. First, initial infection with this virus usually occurs in infancy and once infected it remains lifelong in the peripheral nervous system (PNS) in a latent state. However, HSV1 can be reactivated repeatedly by events such as stress and immunosuppression, leading to a productive infection and virus replication, and in some people this results in herpes labialis (cold sores). Thus, if HSV1 were eventually to reach the brain, repeated reactivation of the virus there could lead to accumulation of damage, manifesting at a late stage in life, consistent with the onset of AD usually in older age. Second, the virus is ubiquitous, infecting about 90% of the adult population: a necessary characteristic in view of the high prevalence of AD. Finally, HSV1 causes a rare but severe brain disorder, herpes simplex encephalitis (HSE), and the main regions affected, the frontal and temporal cortices, are those showing the main pathological changes in AD; for these reasons, the virus was proposed as a likely candidate agent in AD [Ball, 1982]. Further, those who survive HSE usually suffer from memory loss and cognitive impairment [Hokkanen and Launes, 2000].
This review focuses on the questions that have been asked in order to investigate a possible role for HSV1 in AD (but omits descriptions of the virus lifecycle and of certain viral effects that may play a role, such as oxidation and autophagy, as they were discussed in a previous review [Itzhaki and Wozniak, 2008]). Further, it describes the use of current and of possible future antiviral agents.
Is HSV1 Present in Elderly Human Brains?
Although HSV1 could exert its influence on the brain indirectly (from the PNS) or operate via a hit-and-run mechanism, it is probable that if the virus has a role in AD it does so by causing damage whilst in the brain. Therefore, to investigate its possible role, it was necessary first to establish whether HSV1 is present in the brain in normal circumstances (i.e. other than during HSE). Using the ultrasensitive method of solution polymerase chain reaction (PCR), a high proportion of elderly people, including AD patients, were found to have HSV1 DNA residing, in latent form, in their brain [Jamieson et al. 1991]. Consistent with the tropism the virus exhibits in HSE, and with the regions exhibiting pathology in AD, the viral DNA was found in the temporal and frontal cortices. Since then, five other groups have broadly substantiated this finding using solution PCR [Rodriguez et al. 2005; Mori et al. 2004; Gordon et al. 1996; Baringer and Pisani, 1994; Bertrand et al. 1993]. Subsequently an immunological method confirmed that the virus was present in brain and showed also that it had replicated there, causing a productive infection, perhaps recurrently. This was done by demonstrating an HSV1-specific intrathecal antibody response in AD sufferers and elderly controls [Wozniak et al. 2005], and was based on the finding that after HSE, antibodies to the virus can be detected in the CSF up to several years later [Skoldenberg et al. 1981]. More recently, in situ PCR has further confirmed HSV1 DNA presence in brains of AD patients and age-matched normal subjects [Wozniak et al. 2009b]. Interestingly, all of these methods detected HSV1 only rarely in the brain of younger people [Wozniak et al. 2005, 2009b; Jamieson et al. 1992], suggesting that it reaches the brain in older age, perhaps due to the decline in the immune system [Gouin et al. 2008; Stowe et al. 2007].
It could be argued that if an infectious agent is a factor in AD, it should be possible, using postmortem brain specimens that harbour the infection, to culture it or transmit it to a new host. However, HSV1 in brain is presumably latent most of the time (otherwise the patient would be suffering from HSE), and in this state it is not infectious, as only the viral genome is present and only one set of transcripts appears to be made. Therefore, the virus would need to be reactivated for culturing or transmission, and as very little work has been done on reactivation of HSV1 in or from brain, even with mice, and probably none from human brains (where a major problem might be post-mortem delay), this is not feasible at present.
Does a Genetic Factor Act with HSV1 to Confer a Strong Risk of AD?
The presence of HSV1 in brains of elderly controls as well as AD patients does not preclude a major role for the virus in AD. The extent of damage caused by many, if not all, infectious agents is determined by host factors. For example, cold sores occur in only 20–40% of the population, despite the high prevalence of HSV1 and the fact that it reactivates in everybody infected; presumably their occurrence is determined by a host factor(s). Similarly, the tuberculosis bacterium, Mycobacterium tuberculosis, infects 10 times more people than it actually affects, i.e. far more people are infected than affected. In other words, 'controls' can be asymptomatic carriers. A similar phenomenon was postulated to occur in AD and in fact HSV1 was found to confer a high risk of AD when in the brain of people who possess a specific genetic factor, the type 4 allele of the apolipoprotein E gene (APOE-ε4) [Lin et al. 1998; Itzhaki et al. 1997a]. (APOE codes for the protein, apoE, which exists in three major isoforms, types 2, 3, and 4; it is involved in the transport of lipids in the body and in repair of tissue damage.) This joint genetic–environmental risk factor applied to 60% of the cases examined; presumably, other factors apply to the remaining 40%. The results showed that AD brain is not predisposed to HSV1 infection (the proportions of elderly controls and of AD patients harbouring HSV1 in brain are similar) nor does APOE-ε4 carriage predispose to HSV1 infection (very few brain-infected elderly controls carry an e4 allele). Intriguingly and consistently, APOE-ε4 was found to be a risk for cold sores [Lin et al. 1998; Itzhaki et al. 1997a].
Our data are supported by the studies of Itabashi and colleagues, who found a statistically significant association between HSV1 presence in brain and APOE-ε4 carriage in AD patients [Itabashi et al. 1997] (interestingly, this was so despite the relatively low proportion who were HSV1-positive in brain, consistent with the lower HSV1 infection level in Japan). Also, Beffert and coworkers showed a trend towards association [Beffert et al. 1998]. In contrast, two groups found no association between HSV1 and APOE-ε4 in AD [Hemling et al. 2003; Marques et al. 2001], but as each detected HSV1 DNA in only one brain (out of 34 and 15, respectively), any such link could not have been revealed. The reason for the low proportion of HSV1-positive brains is unclear. Both groups appeared to have carried out most of the many checks essential for PCR usage, but in the case of the US study, certain procedural difference might have accounted for the low detection level [Itzhaki et al. 2001]; in the case of the Finnish study the authors suggested [Hemling et al. 2004] that the differences might relate to the different populations studied.
How might HSV1 and apoE interact? One possibility, supported by subsequent studies on APOE and several infectious diseases, is that they might compete for binding and entry at the cell membrane [Itzhaki et al. 1997b], where both virus and protein bind to heparan sulfate proteoglycans (HSPG) before attaching to specific receptors for cell entry [Ji et al. 1993; WuDunn and Spear, 1989]. If apoE4 were to compete less than the other isoforms, more virus could enter, spread, and cause damage, leading to AD. In fact, APOE genotype governs the outcome of infection by several diverse infectious agents that use HSPG and/or specific apoE receptors for binding and entry, including HSV2, HIV and HCV [Itzhaki and Wozniak, 2009; Jayasuriya et al. 2008; Wozniak et al. 2002, 2003, 2007b; Lin et al. 2001; Corder et al. 1998].
Does APOE Affect HSV1 Expression and Load in Brain?
Recently, three groups have used APOE-transgenic mice to find whether, on HSV1 infection, there are isoform-specific effects of APOE on viral load, or on viral expression. All of the studies showed that carriage of APOE-ε4 led to potentially more damaging effects than carriage of APOE-ε2 or ε3: the first found a greater viral load in the brains of productively and latently infected APOE-ε4 mice [Burgos et al. 2003, 2006]; the second showed that on infection of cultured foetal neurons from APOE-transgenic mouse brains, expression of HSV1 immediate–early genes was greater in cells from e4 animals, and in APOE-ε4 mice, latency was established later [Miller and Federoff, 2008]; the third found that the viral load was greater in trigeminal ganglia and brain of latently infected APOE-ε4 mice [Bhattacharjee et al. 2008]. All of these studies thus support the proposal that HSV1 infection is indeed modulated by APOE, with greater viral damage occurring in brain of APOE-ε4 carriers, resulting in AD.
Is There an HSV1 Connection with Senile Plaques and/or β-amyloid?
The brains of AD sufferers are characterized by an abundance of two abnormal features: senile plaques (SPs) and neurofibrillary tangles (NFTs). It is these features that are thought to be central to disease pathogenesis, despite also being present in the brains of elderly normal subjects, although usually at lower levels. SPs have many components but they comprise mainly a peptide called β-amyloid (Aβ), which is generated by the enzymatic cleavage of a much longer protein called amyloid precursor protein (APP). The enzymes involved in APP processing are termed secretases; β- and γ-secretases are necessary for Aβ formation, but although α-secretase can cleave APP, its action precludes the formation of Aβ. Interestingly, γ-secretase also processes nectin-1 [Kim et al. 2002], a member of the immunoglobulin superfamily involved in synapse formation, that is the main receptor on neurons and epithelial cells for HSV1 [Stiles et al. 2008]. In addition, current studies by S. Lim et al. (Georgetown University, personal communication) suggest that β-secretase is also involved with nectin-1: nectin-1 and β-secretase colocalize at synapses, and the interaction of nectin-1 with the virus affects the processing of the former.
Despite a vast amount of research on Aβ, nothing has been revealed about the underlying cause(s) of its overproduction in sporadic AD. However, recent evidence suggests that HSV1 has a direct role in its generation. Immunocytochemistry and enzyme-linked immunosorbent assay (ELISA) have revealed that HSV1 infection of human neural-type cells (neuroblastoma and glioblastoma) in culture causes a striking increase in intracellular level of Aβ [Wozniak et al. 2007a]. Interestingly, it is now thought that intracellular formation of Aβ, rather than extracellular deposition of Aβ, is an early event in AD. Also, immunohistochemistry showed an appreciable amount of Aβ in the brains of HSV1-infected mice [Wozniak et al. 2007a]. Consistently, β- and γ-secretase levels increased in HSV1-infected neural cells [Wozniak et al. 2007a]. These findings significantly extend early work by Cribbs and colleagues and by Satpute-Krishnan and coworkers demonstrating indirect links between HSV1 and Aβ. Cribbs and colleagues showed that synthetic peptides derived from an HSV1 glycoprotein not only accelerate the formation of Aβ fibrils in vitro and are neurotoxic at doses similar to those of Aβ, but also they self-assemble into fibrils ultrastructurally indistinguishable from Aβ [Cribbs et al. 2000]. These data suggest that HSV1 might 'seed' plaque formation [Cribbs et al. 2000]. Satpute-Krishnan and coworkers found that APP is associated with HSV1 during anterograde transport of the virus [Satpute-Krishnan et al. 2003], which might affect APP degradation and synaptic function. Interestingly, other infectious agents can cause Aβ accumulation, including HIV [Esiri et al. 1998], HSV2 [Wozniak, Dobson and Itzhaki, unpublished], West Nile virus [Dhingra et al. 2005], Chlamydia pneumoniae [Little et al. 2004], and Borrelia burgdorferi spirochetes [Miklossy et al. 2006]. However, unlike these other agents, HSV1 is present in a high proportion of elderly brains and is thus uniquely positioned to contribute to AD neuropathology.
The reason why HSV1 causes Aβ accumulation is unknown. In general, the virus decreases the synthesis of host cell proteins, apart from those that it requires for its replication. It therefore seems paradoxical that the virus increases Aβ level in cell culture and mouse brains. One possibility is that these changes are required for the synthesis of progeny viruses. Interestingly, Aβ fibrils have been shown to enhance infection of several enveloped viruses including HSV1 [Wojtowicz et al. 2002]. Alternatively, cells might increase Aβ as part of their defence response, and the fact that certain other infectious agents, and also chemicals (such as hydrogen peroxide and mercury which, like HSV1, cause oxidative damage) cause Aβ deposition, suggests that Aβ production might be a general response to infection and to certain types of chemical damage. Aβ might initially entomb the agent (at least in the case of pathogens), thereby preventing further damage to the host, but eventually, through overproduction, result in toxicity via oligomer formation. In fact, there is evidence that amyloid oligomers have bacteriostatic activity [R. Tanzi, personal communication; Soscia et al. 2010; Strobel, 2009]. SP in human brain might therefore represent a marker of infection in the brain.
Most excitingly, a combination of in situ PCR, which detects a specific DNA sequence, if present, in sections of tissue (thus revealing its distribution in tissue), with immunohistochemistry for Aβ or thioflavin S staining for SP has shown that HSV1 DNA is located very specifically within plaques in human frontal and temporal cortex [Wozniak et al. 2009b] (Figure 1). Ninety per cent of the plaques contain HSV1 DNA, and in AD brain, 72% of the viral DNA is associated with plaques (only 24% in elderly normal brains, perhaps reflecting a lesser production or a greater clearance of amyloid). It might be argued that the localization of the viral DNA within plaques is artefactual or incidental, but many facts vitiate this. First, preexisting plaques might attract and engulf viral DNA from other sites in the brain, but this is unlikely as viral DNA is large (which would hinder movement), and does not persist for long periods. Second, microglia, which are known to be attracted to plaques, might engulf HSV1 DNA or be infected by the virus and then carry the viral DNA to plaques. Again this is unlikely as HSV1 is rarely detected in these cells [Esiri et al. 1995]. Third, preexisting plaques might reactivate HSV1 through their known inflammatory effects. However, this would require 80–90% of plaques (the percentage that contain viral DNA) to be situated immediately adjacent to latently infected cells, which would be an astonishing coincidence. Finally, the viral DNA in plaques might result merely from adherence to the plaque surface, which is known to be 'sticky'. This is also improbable as no cell DNA has been detected in plaques [Ginsberg et al. 1997] (despite being present in vastly greater amounts than viral DNA), and in some 10–20% of plaques we examined, there is no viral DNA whatsoever. Also, the average size of plaques is about 50 mm in diameter but our sections are only 7 mm thick; thus, most sections must display the interior of plaques and they show that viral DNA is present throughout, rather than merely on their surface.
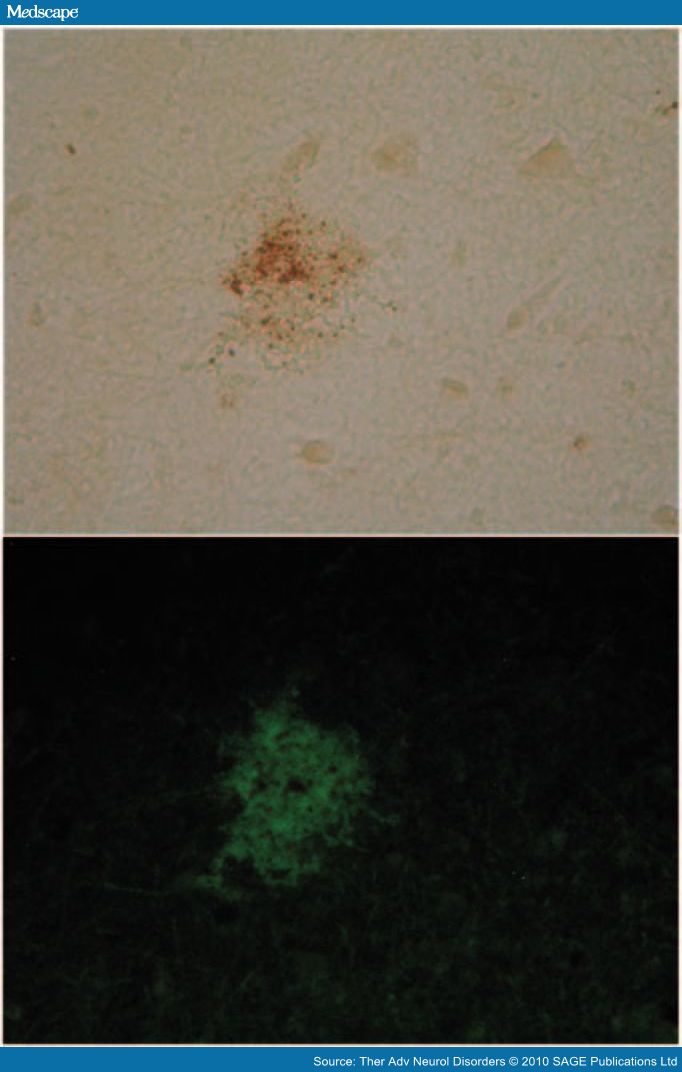
Figure 1. Herpes simplex virus type 1 (HSV1) DNA is localized in senile plaques of Alzheimer's disease brain. In situ polymerase chain reaction (PCR) was used to locate the HSV1 DNA in sections of human brain. In this method, which is far more sensitive than in situ hybridization, a digoxigenin-labelled probe is hybridized to the target sequence in the tissue section after PCR amplification; it thus allows simultaneous amplification and localization. To locate plaques, thioflavin S staining was used. Sections were viewed under fluorescent light to locate the plaques (green staining) and then under white light to reveal the HSV1 DNA (brown staining).
Our in situ PCR findings, together with the increased deposition of Aβ after infection [Wozniak et al. 2007a], strongly support a causal role for HSV1 in the formation of plaques and of putative toxic amyloid products.
Is There an HSV1 Connection with Abnormally Phosphorylated Tau?
The other important neuropathological feature of AD, NFT, comprises mainly abnormally phosphorylated and hyperphosphorylated forms of a protein called tau. Tau is a microtubuleassociated protein involved in intracellular transport and when it is phosphorylated, transport is impaired. Like Aβ, several micro-organisms can cause tau phosphorylation or NFT formation: tau hyperphosphorylation occurs in cells infected with Borrelia burgdorferi spirochetes [Miklossy et al. 2006]; hyperphosphorylated tau occurs in the brains and CSF of HIV-infected individuals [Anthony et al. 2006]; and NFT occur in the brains of subacute sclerosing panencephalitis sufferers, years after the initial infection with measles virus [Bancher et al. 1996; McQuaid et al. 1994].
Recently, immunocytochemistry, ELISA and Western blotting have also shown that HSV1 infection of cultured human neural cells caused abnormal and hyperphosphorylation of several sites in tau which are characteristic of tau in AD brain [Wozniak et al. 2009a]. Further, the enzymes responsible for phosphorylation at these sites, protein kinase A (PKA) and glycogen synthase kinase 3β (GSK 3β), increased on infection [Wozniak et al. 2009a]. In addition, Zambrano and colleagues showed that tau hyperphosphorylation, altered microtubule dynamics and neurite damage occur in HSV1-infected cultures of cortical neurons from foetal mouse [Zambrano et al. 2008].
As with Aβ, the reason why abnormal tau phosphorylation occurs during HSV1 infection is unknown but it could be a cellular defence mechanism or it might be required for viral replication. Supporting the latter suggestion is the fact that lithium, an inhibitor of tau phosphorylation (via inhibition of GSK3β) [Avila and Hernandez, 2007], inhibits HSV1 infection too [Amsterdam et al. 1996].
Are There HSV1 Connections with AD-relevant Genes?
As well as its association with APOE-ε4, HSV1 might interact with several other AD susceptibility genes [Carter, 2008]. Of particular interest is the recent identification of polymorphisms in the gene for protein kinase R (PKR) that confer greater risk of AD. PKR is part of a cellular defence mechanism against viruses. When PKR is activated it phosphorylates the β subunit of the eukaryotic initiation factor (eIF2β), which is required for translation. Phosphorylation of eIF2β leads to inhibition of most protein synthesis, which should benefit the cell by limiting also viral protein synthesis and hence its replication. Therefore, polymorphisms in PKR that affect the host response to infection might well lead to increased damage by viruses, particularly HSV1 [Bullido et al. 2008]. A further consequence of eIF2β phosphorylation by PKR is that it causes the release of the translation brake on the β-secretase gene (normally, this gene is tightly regulated) [G. Ill-Raga and F.J. Muñoz, Universitat Pompeu Fabra, Barcelona, personal communication]. Thus, the increase in β-secretase and consequent increase in Aβ that occurs during HSV1 infection might occur because of PKR activation and eIF2α phosphorylation and might be part of the cell's defence mechanism.
Another gene that might be involved in AD and which has links with HSV1 is the major AD susceptibility gene found near chromosome 14q31. This gene, the identity of which is currently unknown, could be the IgGM locus, which is located on chromosome 14q32 [Pandey, 2009]. This suggestion relates to the ability of HSV1 to interfere with the function of IgG molecules. HSV1 encodes two glycoproteins (gE and gI) that form a heterodimer which is able to bind the Fc region of IgG molecules. When this occurs, the IgG molecule is impaired in its function and so cannot fight the virus. Therefore, any mutation in the Fc region of IgG that facilitates its binding to the gE–gI heterodimer would have detrimental effects on the host by enhancing HSV1 infection. The gE–gI heterodimer of HSV1 binds more strongly to IgG molecules that have the IgGM 1, 17 mutation than IgG molecules with the IgGM 3 mutation. Thus, people with the IgGM 1, 17 mutation could not fight HSV1 infection as well, so more virus particles would be available to cause damage, leading to AD, suggesting that the IgGM 1, 17 allele would be more prevalent among AD patients.
Is Latent HSV1 in Brain Activated by Peripheral Infection?
Reactivation of HSV1 from the PNS is stimulated by several factors, including ultraviolet light exposure and fever. These stimuli are thought to trigger HSV1 reactivation via the production of inflammatory cytokines. Little work has been done on the stimuli that trigger HSV1 reactivation in the brain but one possibility might be peripheral infections: these produce inflammatory cytokines which cause activation of microglia within the brain. These activated microglia produce additional cytokines that could lead to HSV1 reactivation in the brain (inflammation is known to reactivate latent HSV1). Thus, one might predict that people who have more frequent or more extensive peripheral infections would have increased reactivation of HSV1 in brain and therefore might have a greater risk of developing cognitive decline or even AD. In fact, several epidemiological studies have shown an association between extent or number of infectious episodes and cognitive decline in the elderly. In one case, seropositivity for herpesviridae, APOE-ε4 carriage and low education level in elderly cardiovascular patients were found to be associated with risk of cognitive impairment [Strandberg et al. 2003]. In another study, cognitive decline in AD patients was shown to occur for at least 2 months after systemic infection [Holmes et al. 2003]. A third study, which surveyed a group of people aged ≥65 over a 14-year period, suggests that those who experience HSV1 reactivation have an increased risk of developing AD [Letenneur et al. 2008]. At the start of the 14 years, serum anti-HSV1 IgM was investigated as a marker of recent HSV1 reactivation in the PNS. Those who were IgM-positive showed a significantly higher risk of developing AD in subsequent years than those who were IgM-negative. As the stimuli that cause HSV1 reactivation in the PNS are very likely also to cause HSV1 reactivation in brain, the subjects who displayed PNS reactivation (as shown by serum IgM) might well have suffered its reactivation in brain too. These data therefore provide not only another example of a peripheral infection influencing the brain, but also suggest that IgM-positive individuals are at greater risk of AD because of the reactivation of the HSV1 present in their brains. The study showed no dependence of occurrence of reactivation on APOE. This was consistent with our data on CSF and serum [Wozniak et al. 2005] revealing the presence of intrathecal IgG in some of our subjects, which demonstrated clearly that HSV1 had reactivated in their brains, and which showed that the intrathecal IgG occurrence was independent of APOE genotype. We therefore suggested that APOE might determine the extent of damage caused on reactivation (and/or the extent of repair), rather than occurrence or frequency of reactivation.
It might be argued that the inflammation in brain that follows peripheral infection or stress directly leads to oligomer and plaque formation and is, on its own, the main cause of AD, with HSV1 being merely a bystander. However, our discoveries that HSV1 can reactivate in brain, possibly recurrently, causing an acute infection, that acute HSV1 infection causes Aβ accumulation and that in AD brains viral DNA is located very specifically in amyloid plaques, suggest that HSV1 has a direct role in the development of AD, presumably via the formation of toxic amyloid oligomers.
Antiviral Treatment for AD?
The involvement of HSV1 in AD suggests that antiviral agents might prevent further deterioration of patients, and possibly, in the future, vaccination against HSV1 might prevent the development of AD. Future clinical trials should investigate the usage of antiherpetics such as acyclovir (ACV), and its biodrug valacyclovir (which is converted to ACV), in AD sufferers. ACV crosses the normal blood–brain barrier and causes few side effects, apart from in patients with renal impairment. ACV has been used for treating MS patients (based on the putative role of another herpes virus in MS) and a clinical trial showed that it crossed the blood–brain barrier, that no patients demonstrated a damaged barrier [Lycke et al. 2003] and that even a dosage of 3 g per day for 2 years caused no visible ill effects [Friedman et al. 2005].
ACV is a nucleoside analogue that stops viral replication. Its action depends on the presence of HSV1 thymidine kinase (TK). TK phosphorylates ACV to its monophosphate form, and then cell enzymes further phosphorylate it to the diphosphate and triphosphate forms. The triphosphate competes with dGTP as a DNA polymerase substrate and so it prevents chain elongation in newly forming DNA. Thus, ACV 'finds' HSV1-infected cells and stops virus replication.
Another possible agent is intravenous immunoglobulin (IVIG), which acts by a completely different mechanism from that of ACV and other anti-HSV1 antiviral agents in current use for acute HSV1 disorders such as HSE (and cold sores). IVIG products are derived from the pooled plasma of thousands of people, so they contain large amounts of neutralizing antibodies to a number of microbes, including HSV1. IVIG is able not only to neutralize any extracellular virus but also to help destroy cells acutely infected with HSV1 [Kohl and Loo, 1986]: a useful feature as the virus can be transferred from cell to cell without release of extracellular virus. In murine models of HSE, IVIG was found to protect against death and it reduced the number of trigeminal ganglia containing latent HSV1 [Erlich and Mills, 1986]. Further, a study of genital herpes in humans showed that the reduction in recurrence frequency and duration was far greater, and the lesion severity less, with IVIG treatment than with ACV, and there was a trend towards IVIG causing a greater reduction in viral load [Masci et al. 1995].
One potential problem with the use of IVIG in AD is that it might have only limited access to the brain, but there is evidence that the blood–brain barrier is permeable in AD [Pahnke et al. 2009] and so it is likely that IVIG would enter AD patients' brains. In fact, IVIG has been tried in a few AD patients for a totally different purpose, namely, to augment their relatively low level of anti- Aβ antibodies, with the aim of enhancing the clearance of Aβ. A very small-scale retrospective study of patients treated with 0.4 g/kg every 2 weeks for 3.5–6 months found that IVIG was well tolerated, and that neurocognitive test scores were either stable or otherwise showed trends toward improvement in some aspects [Devi et al. 2008]. A subsequent equally small study of patients with mild AD showed that anti- Aβ antibodies in serum increased proportionately to IVIG dose, and that 'mini-mental state' scores increased during treatment [Relkin et al. 2009]; interestingly, use of monoclonal anti- Aβ antibodies did not lead to similar encouraging effects on cognitive function, suggesting perhaps that the IVIG had affected some factor other than Aβ. Further, a recent retrospective study has shown that prior treatment with IVIG (at least once during a 3-year period) reduces the risk of developing AD in patients aged 65 years or over, compared with untreated controls (although over 30% of those treated with IVIG had cancer diagnoses, and so their lower risk of AD might have reflected their associated treatments and diagnoses [Fillit et al. 2009]). It is unknown whether in these studies there was an IVIG-induced anti- HSV1 action (especially in APOE-ε4 carriers, as it is the combination of HSV1 in brain and APOE-ε4 that confers risk of AD, but unfortunately no data on APOE genotypes were presented). However, an anti-HSV1 action might explain the apparent paradox that monoclonal Aβ did not have the same beneficial effect as IVIG.
Currently available anti-HSV1 antiviral agents work by stopping viral DNA synthesis and therefore they prevent only the damage caused by the virus that is initiated as a consequence of viral DNA synthesis. Thus, they might be ineffective against damage occurring independently of viral DNA synthesis, for example damage resulting from viral attachment to the cell membrane or viral entry into the cell. However, we have found that the HSV1-induced formation of Aβ and of AD-like tau occurs only after synthesis of the first group of viral proteins following viral entry: the so-called immediate–early (IE) proteins [Wozniak, Frost, Preston, and Itzhaki, unpublished observations]. Consistently, preliminary experiments with ACV show that its addition to HSV1-infected cell cultures does indeed decrease greatly the levels of Aβ and of AD-like tau in the cells (as well as viral replication, as expected).
Concluding Remarks
The evidence detailed above strongly supports a causal role for HSV1 in AD. The cascade of events leading to AD might involve reactivation of latent HSV1 in brain by events such as stress and peripheral infection, resulting in a productive but localized infection: possibly a 'mild', atypical encephalitis. (Cases of mild and of recurrent HSE have been reported: these perhaps occur relatively often but are underdiagnosed [Tyler et al. 1995; Klapper et al. 1984].) Infection would cause both direct damage and indirect, inflammatory-mediated damage, and in APOEe4 carriers the damage would be greater, possibly through greater viral replication and spread, eventually leading to AD. The mechanism might well involve viral-induced increases in Aβ and AD-like tau, both of which have been strongly implicated in the aetiology of the disease; such increases might initially be cellular defence mechanisms but eventually, through uncontrolled production, they would cause cell damage or death (Figure 2).
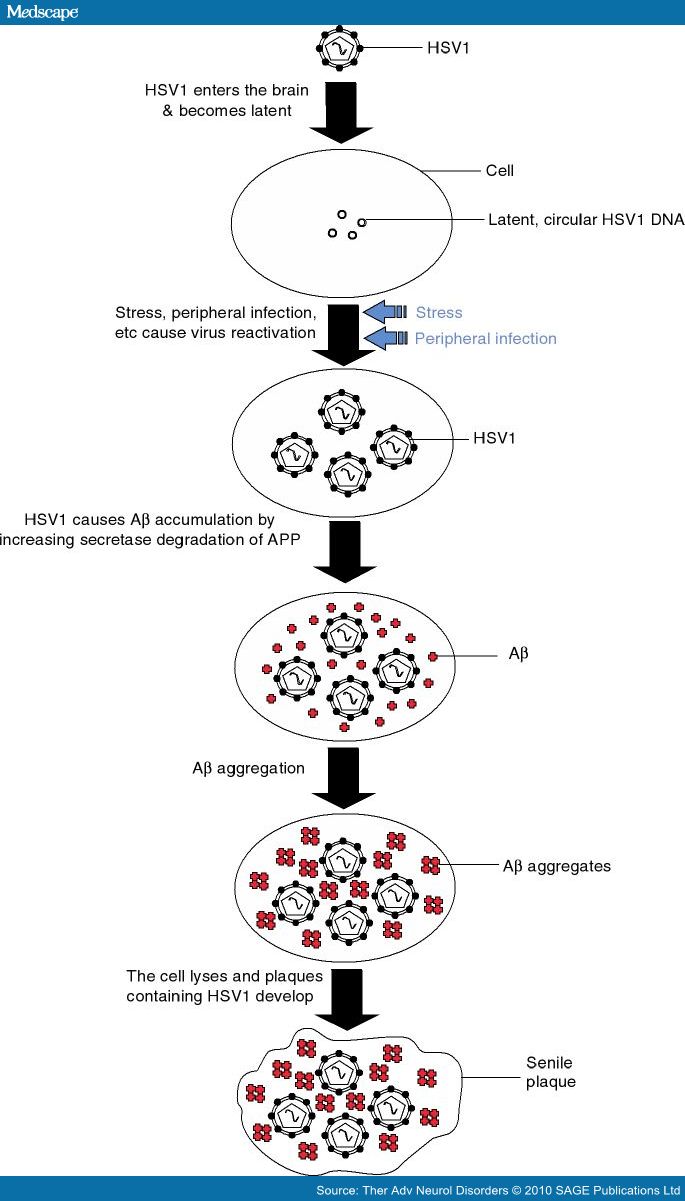
Figure 2. Scheme showing how herpes simplex virus type 1 (HSV1) might cause senile plaques. HSV1 enters the brain in later life and becomes latent. Events such as stress and peripheral infection cause the virus to reactivate and the resulting productive infection causes β-amyloid (Aβ) to be produced which then aggregates. Eventually the cell is destroyed and the Aβ aggregates plus cellular debris form extracellular senile plaques which contain also HSV1 DNA.
Antiviral agents, unlike other treatments that are available or are being tested, would provide a completely new approach in that they would inhibit a major cause of the disease rather than inhibiting the consequences/symptoms (Aβ, Aβ oligomers or AD-like tau). In addition, by targeting a cause of AD, not only would Aβ, Aβ oligomers or AD-like tau be prevented but other products that might be involved in AD pathogenesis would also be inhibited.
References
- Amsterdam, J.D., Maislin, G. and Hooper, M.B. (1996) Suppression of herpes simplex virus infections with oral lithium carbonate—a possible antiviral activity. Pharmacotherapy 16: 1070–1075.
- Anthony, I.C., Ramage, S.N., Carnie, F.W., Simmonds, P. and Bell, J.E. (2006) Accelerated Tau deposition in the brains of individuals infected with human immunodeficiency virus-1 before and after the advent of highly active anti-retroviral therapy. ActaNeuropathol (Berl) 111: 529–538.
- Avila, J. and Hernandez, F. (2007) GSK-3 inhibitors for Alzheimer's disease. Expert Rev Neurother 7: 1527–1533.
- Ball, M.J. (1982) Limbic predilection in Alzheimer dementia: is reactivated herpesvirus involved? Can JNeurol Sci 9: 303–306.
- Bancher, C., Leitner, H., Jellinger, K., Eder, H., Setinek, U., Fischer, P. et al. (1996) On the relationship between measles virus and Alzheimer neurofibrillary tangles in subacute sclerosing panencephalitis. Neurobiol Aging 17: 527–533.
- Baringer, J.R. and Pisani, P. (1994) Herpes simplex virus genomes in human nervous system tissue analyzed by polymerase chain reaction. Ann Neurol 36: 823–829.
- Beffert, U., Bertrand, P., Champagne, D., Gauthier, S. and Poirier, J. (1998) HSV-1 in brain and risk of Alzheimer's disease. Lancet 351: 1330–1331.
- Bertrand, P., Guillaume, D., Hellauer, L., Dea, D., Lindsay, J., Kogan, S. et al. (1993) Distribution of herpes simplex virus type 1 DNA in selected areas of normal and Alzheimer's disease brains: a PCR study. Neurodegeneration 2: 201–208.
- Bhattacharjee, P.S., Neumann, D.M., Foster, T.P., Bouhanik, S., Clement, C., Vinay, D. et al. (2008) Effect of human apolipoprotein E genotype on the pathogenesis of experimental ocular HSV-1. Exp EyeRes 87: 122–130.
- Bullido, M.J., Martinez-Garcia, A., Tenorio, R., Sastre, I., Munoz, D.G., Frank, A. et al. (2008) Double stranded RNA activated EIF2 alpha kinase (EIF2AK2; PKR) is associated with Alzheimer's disease. Neurobiol Aging 29: 1160–1166.
- Burgos, J.S., Ramirez, C., Sastre, I., Bullido, M.J. and Valdivieso, F. (2003) ApoE4 is more efficient than E3 in brain access by herpes simplex virus type 1. Neuroreport 14: 1825–1827.
- Burgos, J.S., Ramirez, C., Sastre, I. and Valdivieso, F. (2006) Effect of apolipoprotein E on the cerebral load of latent herpes simplex virus type 1 DNA. J Virol 80: 5383–5387.
- Carter, C.J. (2008) Interactions between the products of the Herpes simplex genome and Alzheimer's disease susceptibility genes: relevance to pathologicalsignalling cascades. Neurochem Int 52: 920–934.
- Corder, E.H., Robertson, K., Lannfelt, L., Bogdanovic, N., Eggertsen, G., Wilkins, J. et al. (1998) HIV-infected subjects with the E4 allele for APOE have excess dementia and peripheral neuropathy. Nat Med 4: 1182–1184.
- Cribbs, D.H., Azizeh, B.Y., Cotman, C.W. and LaFerla, F.M. (2000) Fibril formation and neurotoxicity by a herpes simplex virus glycoprotein B fragment with homology to the Alzheimer's A beta peptide. Biochemistry 39: 5988–5994.
- Devi, G., Schultz, S., Khosrowshahi, L., Agnew, A. and Olali, E. (2008) A retrospective chart review of the tolerability and efficacy of intravenous immunoglobulin in the treatment of Alzheimer's disease. J AmGeriatr Soc 56: 772–774.
- Dhingra, V., Li, Q., Allison, A.B., Stallknecht, D.E. and Fu, Z.F. (2005) Proteomic profiling and neurodegeneration in West-Nile-virus-infected neurons. J Biomed Biotechnol 2005: 271–279.
- Erlich, K.S. and Mills, J. (1986) Passive immunotherapy for encephalitis caused by herpes simplex virus. Rev Infect Dis 8 Suppl 4: S439–S445.
- Esiri, M.M., Biddolph, S.C. and Morris, C.S. (1998) Prevalence of Alzheimer plaques in AIDS. J NeurolNeurosurg Psychiatry 65: 29–33.
- Esiri, M.M., Drummond, C.W. and Morris, C.S. (1995) Macrophages and microglia in HSV-1 infected mouse brain. J Neuroimmunol 62: 201–205.
- Fillit, H., Hess, G., Hill, J., Bonnet, P. and Toso, C. (2009) IV immunoglobulin is associated with a reduced risk of Alzheimer disease and related disorders. Neurology 73: 180–185.
- Friedman, J.E., Zabriskie, J.B., Plank, C., Ablashi, D., Whitman, J., Shahan, B. et al. (2005) A randomized clinical trial of valacyclovir in multiple sclerosis. MultScler 11: 286–295.
- Ginsberg, S.D., Crino, P.B., Lee, V.M., Eberwine, J.H. and Trojanowski, J.Q. (1997) Sequestration of RNA in Alzheimer's disease neurofibrillary tangles and senile plaques. Ann Neurol 41: 200–209.
- Gordon, L., McQuaid, S. and Cosby, S.L. (1996) Detection of herpes simplex virus types 1 and 2 and human herpes virus 6 DNA in human brain tissue by polymerase chain reaction. Clin Diagnost Virol 6: 33–40.
- Gouin, J.P., Hantsoo, L. and Kiecolt-Glaser, J.K. (2008) Immune dysregulation and chronic stress among older adults: a review. Neuroimmunomodulation 15: 251–259.
- Hemling, N., Roytta, M., Rinne, J., Pollanen, P., Broberg, E., Tapio, V. et al. (2003) Herpesviruses in brains in Alzheimer's and Parkinson's diseases. AnnNeurol 54: 267–271.
- Hemling, N., Roytta, M., Rinne, J., Pollanen, P., Broberg, E., Tapio, V. et al. (2004) Herpes simplex virus type 1 and Alzheimer's disease. Ann Neurol 55: 300–301.
- Hokkanen, L. and Launes, J. (2000) Cognitive outcome in acute sporadic encephalitis. Neuropsychol Rev 10: 151–167.
- Holmes, C., El-Okl, M., Williams, A.L., Cunningham, C., Wilcockson, D. and Perry, V.H. (2003) Systemic infection, interleukin 1beta, and cognitive decline in Alzheimer's disease. J Neurol Neurosurg Psychiatry 74: 788–789.
- Itabashi, S., Arai, H., Matsui, T., Higuchi, S. and Sasaki, H. (1997) Herpes simplex virus and risk of Alzheimer's disease. Lancet 349: 1102.
- Itzhaki, R.F., Dobson, C.B., Lin, W.R. and Wozniak, M.A. (2001) Association of HSV1 and apolipoprotein E-varepsilon4 in Alzheimer's disease. J Neurovirol 7: 570–571.
- Itzhaki, R.F., Lin, W.R., Shang, D., Wilcock, G.K., Faragher, B. and Jamieson, G.A. (1997a) Herpes simplex virus type 1 in brain and risk of Alzheimer's disease. Lancet 349: 241–244.
- Itzhaki, R.F., Lin, W.R., Shang, D., Wilcock, G.K., Faragher, B. and Jamieson, G.A. (1997b) Herpes simplex virus type 1 in brain and risk of Alzheimer's disease. Lancet 349: 241–244.
- Itzhaki, R.F. and Wozniak, M.A. (2008) Herpes simplex virus type 1 in Alzheimer's disease: the enemy within. J Alzheimers Dis 13: 393–405.
- Itzhaki, R.F. and Wozniak, M.A. (2009) Apolipoprotein E: microbial friend or foe? In: Apoproteins: Research Trends, Nova Science Publications: New York.
- Jamieson, G.A., Maitland, N.J., Wilcock, G.K., Craske, J. and Itzhaki, R.F. (1991) Latent herpes simplex virus type 1 in normal and Alzheimer's disease brains. J Med Virol 33: 224–227.
- Jamieson, G.A., Maitland, N.J., Wilcock, G.K., Yates, C.M. and Itzhaki, R.F. (1992) Herpes simplex virus type 1 DNA is present in specific regions of brain from aged people with and without senile dementia of the Alzheimer type. J Pathol 167: 365–368.
- Jayasuriya, A., Itzhaki, R., Wozniak, M., Patel, R., Smit, E., Noone, R. et al. (2008) Apolipoprotein- {epsilon}4 and recurrent genital herpes in HSV type 2 and HIV co-infected individuals. Sex Transm Infect 84: 516–517.
- Ji, Z.S., Brecht, W.J., Miranda, R.D., Hussain, M.M., Innerarity, T.L. and Mahley, R.W. (1993) Role of heparan sulfate proteoglycans in the binding and uptake of apolipoprotein E-enriched remnant lipoproteins by cultured cells. J Biol Chem 268: 10160–10167.
- Kim, D.Y., Ingano, L.A. and Kovacs, D.M. (2002) Nectin-1alpha, an immunoglobulin-like receptor involved in the formation of synapses, is a substrate for presenilin/gamma-secretase-like cleavage. J Biol Chem 277: 49976–49981.
- Klapper, P.E., Cleator, G.M. and Longson, M. (1984) Mild forms of herpes encephalitis. J Neurol NeurosurgPsychiatry 47: 1247–1250.
- Kohl, S. and Loo, L.S. (1986) In vitro and in vivo antibody-dependent cellular cytotoxicity of intravenous immunoglobulin G preparations against herpes simplex virus. Rev Infect Dis 8 Suppl 4: S446–S448.
- Letenneur, L., Peres, K., Fleury, H., Garrigue, I., Barberger-Gateau, P., Helmer, C. et al. (2008) Seropositivity to herpes simplex virus antibodies and risk of Alzheimer's disease: a population-based cohort study. PLoS ONE 3: e3637.
- Lin, W.R., Graham, J., MacGowan, S.M., Wilcock, G.K. and Itzhaki, R.F. (1998) Alzheimer's disease, herpes virus in brain, apolipoprotein E4 and herpes labialis. Alzheimer's Rep 1: 173–178.
- Lin,W.R.,Wozniak, M.A., Esiri, M.M., Klenerman, P. and Itzhaki, R.F. (2001) Herpes simplex encephalitis: involvement of apolipoprotein E genotype. J NeurolNeurosurg Psychiatry 70: 117–119.
- Little, C.S., Hammond, C.J., MacIntyre, A., Balin, B.J. and Appelt, D.M. (2004) Chlamydia pneumoniae induces Alzheimer-like amyloid plaques in brains of BALB/c mice. Neurobiol Aging 25: 419–429.
- Lycke, J., Malmestrom, C. and Stahle, L. (2003) Acyclovir levels in serum and cerebrospinal fluid after oral administration of valacyclovir. Antimicrob AgentsChemother 47: 2438–2441.
- Marques, A.R., Straus, S.E., Fahle, G., Weir, S., Csako, G. and Fischer, S.H. (2001) Lack of association between HSV-1 DNA in the brain, Alzheimer's disease and apolipoprotein E4. J Neurovirol 7: 82–83.
- Marshall, B.J. and Warren, J.R. (1984) Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1: 1311–1315.
- Masci, S., De Simone, C., Famularo, G., Gravante, M., Ciancarelli, M., Andreassi, M. et al. (1995) Intravenous immunoglobulins suppress the recurrences of genital herpes simplex virus: a clinical and immunological study. Immunopharmacol Immunotoxicol 17: 33–47.
- McQuaid, S., Allen, I.V., McMahon, J. and Kirk, J. (1994) Association of measles virus with neurofibrillary tangles in subacute sclerosing panencephalitis: a combined in situ hybridization and immunocytochemical investigation. Neuropathol Appl Neurobiol 20: 103–110.
- Miklossy, J., Kis, A., Radenovic, A., Miller, L., Forro, L., Martins, R. et al. (2006) Beta-amyloid deposition and Alzheimer's type changes induced by Borrelia spirochetes. Neurobiol Aging 27: 228–236.
- Miller, R.M. and Federoff, H.J. (2008) Isoformspecific effects of ApoE on HSV immediate early gene expression and establishment of latency. NeurobiolAging 29: 71–77.
- Mori, I., Kimura, Y., Naiki, H., Matsubara, R., Takeuchi, T., Yokochi, T. et al. (2004) Reactivation of HSV-1 in the brain of patients with familial Alzheimer's disease. J Med Virol 73: 605–611.
- Pahnke, J., Walker, L.C., Scheffler, K. and Krohn, M. (2009) Alzheimer's disease and blood–brain barrier function—Why have anti-beta-amyloid therapies failed to prevent dementia progression? Neurosci BiobehavRev 33: 1099–1108.
- Pandey, J.P. (2009) Immunoglobulin GM genes as functional risk and protective factors for the development of Alzheimer's disease. J Alzheimers Dis 17: 753–756.
- Relkin, N.R., Szabo, P., Adamiak, B., Burgut, T., Monthe, C., Lent, R.W. et al. (2009) 18-Month study of intravenous immunoglobulin for treatment of mild Alzheimer disease. Neurobiol Aging 30: 1728–1736.
- Rodriguez, J.D., Royall, D., Daum, L.T., Kagan- Hallet, K. and Chambers, J.P. (2005) Amplification of Herpes simplex type 1 and Human Herpes type 5 viral DNA from formalin-fixed Alzheimer brain tissue. Neurosci Lett 390: 37–41.
- Satpute-Krishnan, P., DeGiorgis, J.A. and Bearer, E.L. (2003) Fast anterograde transport of herpes simplex virus: role for the amyloid precursor protein of Alzheimer's disease. Aging Cell 2: 305–318.
- Skoldenberg, B., Kalimo, K., Carlstrom, A., Forsgren, M. and Halonen, P. (1981) Herpes simplex encephalitis: A serological follow-up study. Synthesis of herpes simplex virus immunoglobulin M, A, and G antibodies and development of oligoclonal immunoglobulin G in the central nervous system. Acta Neurol Scand 63: 273–285.
- Soscia, S.J., Kirby, J.E., Washicosky, K.J., Tucker, S.M., Ingelsson, M., Hyman, B. et al. (2010) The Alzheimer's disease-associated amyloid beta-protein is an antimicrobial peptide. PLoS One 5: e9505.
- Stiles, K.M., Milne, R.S., Cohen, G.H., Eisenberg, R.J. and Krummenacher, C. (2008) The herpes simplex virus receptor nectin-1 is down-regulated after transinteraction with glycoprotein D. Virology 373: 98–111.
- Stowe, R.P., Kozlova, E.V., Yetman, D.L., Walling, D.M., Goodwin, J.S. and Glaser, R. (2007) Chronic herpesvirus reactivation occurs in aging. Exp Gerontol 42: 563–570.
- Strandberg, T.E., Pitkala, K.H., Linnavuori, K.H. and Tilvis, R.S. (2003) Impact of viral and bacterial burden on cognitive impairment in elderly persons with cardiovascular diseases. Stroke 34: 2126–2131.
- Strobel, G. (2009) Prague: Aβ Rehabilitated as an Antimicrobial Protein? Alzheimer's Research Forum: http://www.alzforum.org/new/detail.asp?id=2090{2923B2093D2096F-6378-2460B-2098C2053-443363B485284}.
- Tyler, K.L., Tedder, D.G., Yamamoto, L.J., Klapper, J.A., Ashley, R., Lichtenstein, K.A. et al. (1995) Recurrent brainstem encephalitis associated with herpes simplex virus type 1 DNA in cerebrospinal fluid. Neurology 45: 2246–2250.
- Wojtowicz, W.M., Farzan, M., Joyal, J.L., Carter, K., Babcock, G.J., Israel, D.I. et al. (2002) Stimulation of enveloped virus infection by beta-amyloid fibrils. J BiolChem 277: 35019–35024.
- Wozniak, M.A., Faragher, E.B., Todd, J.A., Koram, K.A., Riley, E.M. and Itzhaki, R.F. (2003) Does apolipoprotein E polymorphism influence susceptibility to malaria? J Med Genet 40: 348–351.
- Wozniak, M.A., Frost, A.L. and Itzhaki, R.F. (2009a) Alzheimer's disease-specific tau phosphorylation is induced by herpes simplex virus type 1. J AlzheimersDis 16: 341–350.
- Wozniak, M.A., Itzhaki, R.F., Faragher, E.B., James, M.W., Ryder, S.D. and Irving, W.L. (2002) Apolipoprotein E-epsilon 4 protects against severe liver disease caused by hepatitis C virus. Hepatology 36: 456–463.
- Wozniak, M.A., Itzhaki, R.F., Shipley, S.J. and Dobson, C.B. (2007a) Herpes simplex virus infection causes cellular beta-amyloid accumulation and secretase upregulation. Neurosci Lett 429: 95–100.
- Wozniak, M.A., Mee, A.P. and Itzhaki, R.F. (2009b) Herpes simplex virus type 1 DNA is located within Alzheimer's disease amyloid plaques. J Pathol 217: 131–138.
- Wozniak, M.A., Shipley, S.J., Combrinck, M., Wilcock, G.K. and Itzhaki, R.F. (2005) Productive herpes simplex virus in brain of elderly normal subjects and Alzheimer's disease patients. J Med Virol 75: 300–306.
- Wozniak, M.A., Shipley, S.J., Dobson, C.B., Parker, S.P., Scott, F.T., Leedham-Green, M. et al. (2007b) Does apolipoprotein E determine outcome of infection by varicella zoster virus and by Epstein Barr virus? Eur J Hum Genet 15: 672–678.
- WuDunn, D. and Spear, P.G. (1989) Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J Virol 63: 52–58.
- Zambrano, A., Solis, L., Salvadores, N., Cortes, M., Lerchundi, R. and Otth, C. (2008) Neuronal cytoskeletal dynamic modification and neurodegeneration induced by infection with herpes simplex virus type 1. J Alzheimers Dis 14: 259–269.





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 