Jonathan Himmelfarb, M.D., and T. Alp Ikizler, M.D.
N Engl J Med 2010; 363:1833-1845
Fifty years ago, Belding Scribner and his colleagues at the University of Washington developed a blood-access device using Teflon-coated plastic tubes, which facilitated the use of repeated hemodialysis as a life-sustaining treatment for patients with uremia.1,2 The introduction of the Scribner shunt, as it became known, soon led to the development of a variety of surgical techniques for the creation of arteriovenous fistulas and grafts. Consequently, hemodialysis has made survival possible for more than a million people throughout the world who have end-stage renal disease (ESRD) with limited or no kidney function. The expansion of dialysis into a form of long-term renal-replacement therapy transformed the field of nephrology and also created a new area of medical science, which has been called the physiology of the artificial kidney. This review describes the medical, social, and economic evolution of hemodialysis therapy.
OALS OF HEMODIALYSIS
Dialysis is defined as the diffusion of molecules in solution across a semipermeable membrane along an electrochemical concentration gradient.3 The primary goal of hemodialysis is to restore the intracellular and extracellular fluid environment that is characteristic of normal kidney function. This is accomplished by the transport of solutes such as urea from the blood into the dialysate and by the transport of solutes such as bicarbonate from the dialysate into the blood (Figure 1A)
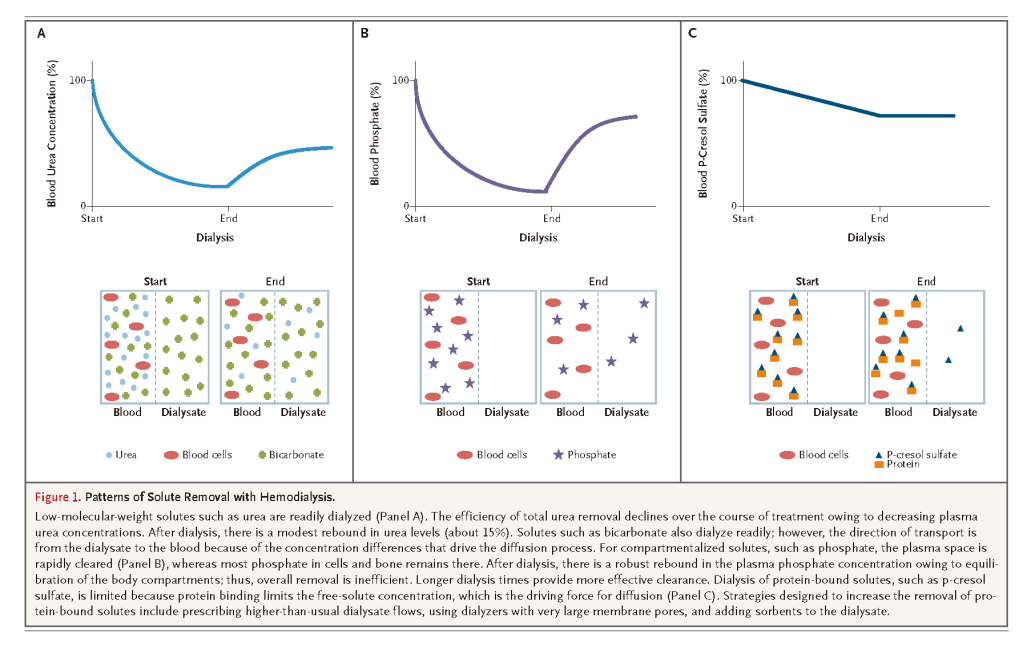
Solute concentration and molecular weight are the primary determinants of diffusion rates. Small molecules, such as urea, diffuse quickly, whereas compartmentalized and larger molecules, such as phosphate, β2-microglobulin, and albumin, and protein-bound solutes, such as p-cresol, diffuse much more slowly (Figure 1B and 1C). In addition to diffusion, solutes may pass through pores in the membrane by means of a convective process driven by hydrostatic or osmotic pressure gradients — a process called ultrafiltration.4 During ultrafiltration, there is no change in solute concentrations; its primary purpose is the removal of excess total body water.
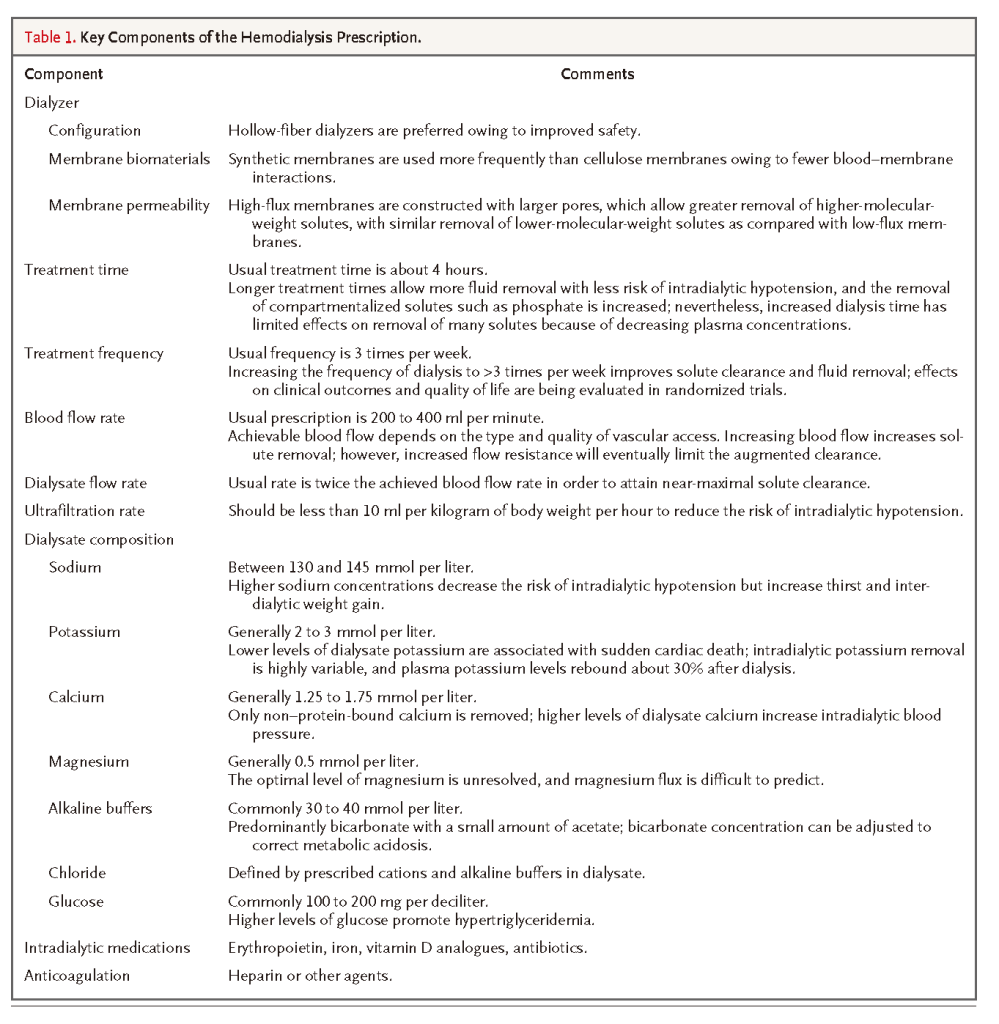
For each dialysis session, the patient's physiological status should be assessed so that the dialysis prescription can be aligned with the goals for the session. This is accomplished by integrating the separate but related components of the dialysis prescription to achieve the desired rates and total amount of solute and fluid removal (Table 1)
By replacing kidney excretory function, dialysis is intended to eliminate the symptom complex known as the uremic syndrome, although ascribing particular cellular or organ dysfunction to the accumulation of specific solutes in uremia has proved to be difficult.5
QUANTIFYING THE DOSE AND ADEQUACY OF DIALYSIS
Measuring the clearance of solutes that accumulate in patients with uremia has become the mainstay for calculating the dose of dialysis and determining its adequacy as delivered. Precise standards and goals of dialysis adequacy are based on the clearance of urea, a byproduct of protein catabolism, which can be readily and accurately measured. The volume of distribution of urea, which is neither lipophilic nor highly protein-bound, reflects total body water; consequently, urea is an attractive molecule for quantifying dialysis adequacy through mathematical modeling based on changing blood concentrations.3,6 Urea kinetic modeling predicts morbidity and mortality better than kinetic modeling of any other known solute. The amount of urea to be removed is usually calculated according to the patient's body size with the use of the following dimensionless construct, which relates the clearance of urea to its volume of distribution in the patient: Kt/Vurea, where K is the urea clearance of the dialyzer, t is the duration of dialysis, and Vurea is the patient's volume of urea distribution. This construct has been readily adopted by the nephrology community to calculate the dialysis dose.6 Some investigators have suggested that adjusting the amount of solute clearance according to the volume of distribution rather than according to the patient's body-surface area may result in underdosing in small patients and women.7-9 Although alternative means of adjusting clearance for body size have been proposed, none currently constitute the standard of care.
The importance of clearance of middle-molecular-weight solutes (500 to 30,000 daltons) with respect to clinical outcomes has long been debated.10 Current high-flux hemodialysis membranes have larger pores than did earlier-generation membranes, and they permit the passage of larger uremic toxins. Since the β2-microglobulin concentration is easy to measure, it is frequently used as a marker solute for middle-molecular-weight solutes. Several retrospective, observational studies have suggested an association between the use of high-flux hemodialysis membranes and reduced mortality.11-14 However, increased clearance of middle-molecular-weight solutes has not been conclusively shown to be an important factor in a well-powered, prospective, randomized trial.
TREATMENT TIME
An important component of the dialysis prescription is treatment time, which can influence the ability to safely remove solutes and accumulated excess fluid. In the 1980s, shortening the treatment time to cut costs while maintaining an adequate level of urea clearance became common practice in the United States. However, subsequent studies revealed that outcomes were adversely affected by shorter treatment times.15 Advocates for longer treatment times pointed to the better outcomes in Europe and Asia, where treatment times are prolonged.16,17 Patients who gain more weight with dialysis are at increased risk for death,18 and a longer treatment time is often required for such patients to help maintain fluid balance. However, to date, little effort has been made to evaluate different fluid-removal strategies in controlled studies.
Reports from individual centers that have used extended dialysis sessions (8 to 12 hours per treatment, often provided overnight) are receiving more attention. Extended treatment times clearly improve blood-pressure control and phosphate removal while having a modest effect on overall solute clearance.19,20 Excellent outcomes have been reported from these centers, although, again, not in the context of randomized clinical trials.21,22 It is unclear whether extended treatments provided at night are practical and would be accepted by most patients undergoing dialysis.
FREQUENCY OF DIALYSIS
For more than four decades, the standard schedule for hemodialysis has continued to be three sessions a week, largely owing to logistic and cost concerns. Although several centers have treated a small number of patients with more frequent hemodialysis, a systematic study of outcomes after such therapy is only now being undertaken. Most available reports are from case–control studies or uncontrolled interventional studies.23 A majority of such studies have shown reductions in blood-pressure levels and in the need for antihypertensive medications, with variable effects on regression of left ventricular hypertrophy, a frequent occurrence among patients receiving long-term hemodialysis. Health-related quality-of-life measures appear to improve with more frequent dialysis treatments, whereas mixed results are reported for measures of anemia control and calcium phosphate metabolism.24 A recent randomized, controlled pilot trial compared daily nocturnal hemodialysis with conventional thrice-weekly hemodialysis.25 In the primary analysis, there was a significant reduction in left ventricular mass in the group treated with daily dialysis, as compared with the conventionally treated group. Improvements in blood-pressure control, serum calcium–phosphorus product, and selected quality-of-life measures were also observed. The Frequent Hemodialysis Network, sponsored by the National Institutes of Health, is currently conducting two studies: in one, daily in-center dialysis involving short treatment times is being compared with conventional thrice-weekly dialysis, and in the other, daily nocturnal dialysis involving longer treatment times is being compared with conventional thrice-weekly dialysis.26Outcomes will include survival, change in left ventricular mass, and quality of life.
EVOLUTION OF HEMODIALYSIS IN THE UNITED STATES
Long-term dialysis was initially available only for patients who were enrolled in a handful of programs (Figure 2)
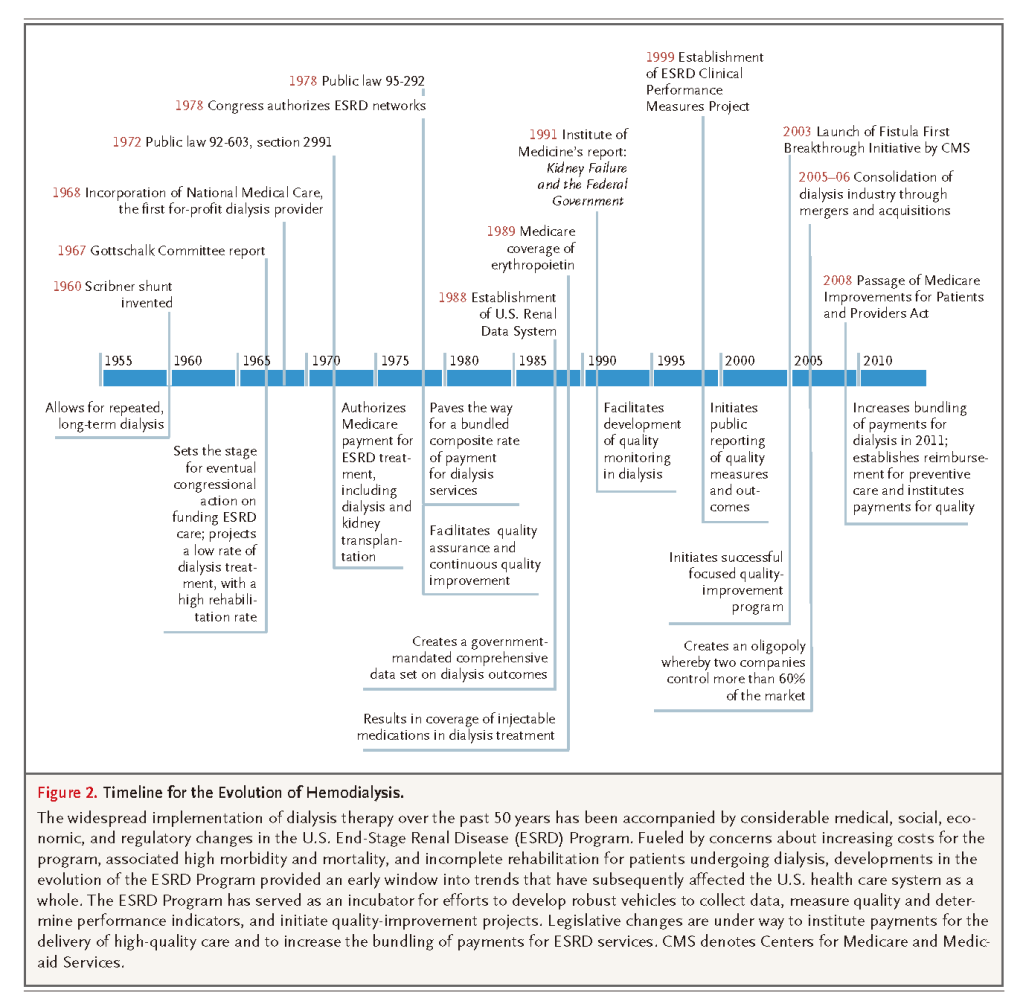
In 1972, President Richard Nixon signed legislation authorizing Medicare coverage for the costs of ESRD treatments, including dialysis and kidney transplantation, for all eligible Americans.27 With little public or congressional debate, the passage of this legislation heralded an era of nearly universal entitlement for ESRD care, in marked contrast to other organ failure–related disease states such as end-stage heart disease or liver disease. Legislators approved the law with the understanding that dialysis would provide high-level rehabilitation and social benefit to a relatively small number of people at low cost.28 Since 1972, a geometric increase in the number of patients receiving dialysis has expanded the scope of the Medicare ESRD Program enormously. From an economic and societal perspective, salient changes during the evolution of this program have included steadily increasing aggregate costs to Medicare, diminished per-treatment reimbursement in inflation-adjusted dollars, a dramatic increase in costs associated with medications given during dialysis, a steady decline in the use of home dialysis (including peritoneal dialysis), and the rise and consolidation of a for-profit dialysis-provider industry.29,30
The demographics of the dialysis population have also changed dramatically over time. Less stringent selection of patients has led to treatment of an increasing proportion of elderly patients, patients with diabetes, and patients who are frail and have complex coexisting conditions. The initiation of dialysis in patients with higher levels of residual kidney function has occurred concomitantly, particularly among patients older than 75 years of age.31 Among elderly nursing-home residents, the initiation of dialysis is associated with a substantial decline in functional status and high mortality.32 The factors driving these clinical practices, and their societal implications, are only beginning to be studied but may well lead to increased consideration of conservative management and palliative-care options for some patients.30,33-35
The Medicare Improvements for Patients and Providers Act (MIPPA) of 2008 contains provisions that are likely to change the Medicare ESRD Program substantially. Currently, dialysis facilities receive a bundled payment for each dialysis session they provide, which includes funds to cover supplies, staffing, and selected ESRD-related laboratory tests; the costs of intravenous medications are billed separately. MIPPA mandates an expansion of the bundled-payment system to include funds for all ESRD-related medications and laboratory tests, beginning in 2011. Including medication reimbursement along with these bundled payments to dialysis providers should remove any possible financial incentive to overprescribe medications during treatment, but simultaneously, it may create incentives for providers to underuse medications or choose to treat patients on the basis of characteristics that may translate into reduced medication expenditures. MIPPA relies on adjustments for case mix to prevent providers from deselecting or cherry-picking patients and also mandates the development of a payment system for quality indicators by 2012.
MEASURING AND IMPROVING QUALITY IN DIALYSIS CARE
The ability to evaluate outcomes among patients with ESRD increased dramatically after 1988, when the United States Renal Data System (USRDS) was established to record and issue reports that would track mortality and morbidity and determine factors affecting clinical outcomes. Perhaps the most robust disease-specific data sets available within the entire Medicare population, these USRDS reports have greatly facilitated the development of quality goals and metrics, at the same time as evidence-based clinical practice guidelines, such as those issued by the Kidney Disease Outcomes Quality Initiative and the Kidney Disease: Improving Global Outcomes program, have been developed.36-38 In 2003 the Centers for Medicare and Medicaid Services (CMS) and other key stakeholders jointly developed a national quality-improvement effort to increase the use of arteriovenous fistulas as the preferred choice for vascular access — a choice that had historically lagged behind other indicators of high-quality care. This collaborative initiative, known as Fistula First, led to a dramatic increase in the use of fistulas.39 In an effort to facilitate patient choice and promote quality improvement, the CMS developed Dialysis Facility Compare, a Web site that allows consumers to compare the mandatory reported performance of dialysis facilities.40
PATIENT SAFETY AND TECHNICAL ADVANCES
Hemodialysis is now substantially safer than it was initially, and deaths directly related to the dialysis procedure are rare. Improved dialysate delivery systems, more reliable monitoring devices, and automated safety mechanisms have reduced the risk of complications. Other technical improvements include the standard use of the more physiologic bicarbonate-based dialysate, better water-quality standards, volumetric ultrafiltration controls, and computer-controlled sodium and potassium modeling.41 Several in-line devices now allow dynamic monitoring of the rate of blood flow through the vascular access,42 changes in the hematocrit (to measure vascular refilling during ultrafiltration), and changes in the electrical conductivity of the dialysate (to estimate the amount of solute being removed).43
Thus, dialysis machines with feedback-control systems currently allow for computer-controlled, real-time adjustments in the critical components of dialysis, such as the ultrafiltration rate.44Automated control of dialysate temperature helps maintain a constant body temperature during dialysis, which may reduce the incidence of intradialytic hypotension.45 Although studies in small groups of patients have suggested possible benefits from in-line monitoring or feedback-control systems, evidence of improved outcomes in large, rigorously controlled trials is lacking.46
TRENDS IN OUTCOMES IN THE UNITED STATES
The steady improvement in procedure-focused and process-related measures of quality has led to a noticeable improvement in survival over the past two decades.36 Nevertheless, the death rate among U.S. patients undergoing dialysis continues to exceed 20% per year during the first 2 years after maintenance dialysis is begun. Unfortunately, hospitalization rates have remained nearly constant, averaging almost 13 hospital days and two admissions per patient-year.47 The exclusive reliance on dialysis-focused quality measures (e.g., adequacy of dialysis, presence or absence of anemia, and mineral metabolism) has previously been questioned, since such measures may account for only 15% of the variations in mortality and morbidity.48 It has been suggested that quality measures also include an assessment of risk factors for cardiovascular disease and infection, which constitute the major causes of hospitalization and death in the population receiving dialysis. Practice patterns also vary greatly, such as variations in the placement and use of fistulas, the rate of coronary revascularization, and the rate of pneumococcal vaccination. Practice-pattern variations in achieving quality-of-care goals, including predialysis care with timely fistula placement, represent a potential area for the improvement of outcomes.
Clinical care of the patient undergoing dialysis is highly complex, given the insidious but protean manifestations of uremia (Table 2)

Although symptoms of uremia are often nonspecific, virtually every organ system in the body is affected by the disruption in metabolic homeostasis associated with ESRD. Physicians who treat patients receiving dialysis must be cognizant of the numerous complications that can result from the loss of kidney function and of the complex relationships between uremia and dialysis treatment. For example, uremia-induced alterations in gastrointestinal tract function can alter nutrient intake and result in poor nutritional status, which in turn increases the risks of cardiovascular disease and infection, particularly when dialysis involves tunneled catheters. Given the problems associated with ESRD, physicians who care for patients receiving hemodialysis face unique and difficult challenges.63 Caring for such patients is particularly difficult because of the lack of high-level evidence in support of target ranges for many of the important components of dialysis care, such as optimal concentrations of parathyroid hormone and low-density lipoprotein (LDL) cholesterol or blood-pressure levels (Table 2).
INTERNATIONAL COMPARISONS
Many investigators have noted that crude mortality rates are consistently higher in the United States than in Europe or Japan. Probably the best available comparative data come from the Dialysis Outcomes and Practice Patterns Study (DOPPS), which uses a prospective design and attempts to harmonize data collection across several countries and continents.64 The DOPPS reported that crude 1-year mortality rates from 1996 to 2002 were 6.6% in Japan, 15.6% in Europe, and 21.7% in the United States.65 Although dramatic differences in demographic characteristics, clinical factors, completeness of data ascertainment, and access to kidney transplantation can limit the validity of these transnational comparisons, the relative risk of death after adjustments have been made for age and multiple coexisting disorders is still higher in the United States than in Japan or Europe.66 Features of practice patterns in the United States that differ from those in the other two countries may account in part for the observed differences in the risk of death. Such features include shorter treatment times, less frequent use of fistulas, and staffing of dialysis units with patient care technicians rather than nurses.67
One trend of concern in the United States is the increasing proportion of patients who begin dialysis with a tunneled catheter rather than with a more permanent type of vascular access. The use of such catheters is strongly associated with increases in the rate of hospitalization, the risk of death, and the cost of care, owing in large part to the risk of catheter-related bacteremia.55 Although the preponderance of available data suggests worse outcomes in the United States, not all investigators agree that differences in practice patterns account for the differences in outcomes. Alternatively, results of a study that used the World Health Organization mortality database suggest that much of the international variation in mortality is attributable to differences in the risk of death that are related to cardiovascular disease in the respective general populations.68
CONTROLLED TRIALS OF DIALYSIS THERAPY
Several randomized, controlled clinical trials with sufficient power to detect changes in mortality or hospitalization rates have evaluated the adequacy of dialysis therapy. The National Cooperative Dialysis Study (NCDS), performed during the 1970s, was designed to determine whether altering the time-averaged concentration of urea or the treatment time — each of which is considered an important determinant of the adequacy of hemodialysis — would affect hospitalization rates.69 The results of the NCDS indicated that a high urea concentration was significantly associated with increased hospitalizations. On the basis of the NCDS results, a minimum delivered dialysis dose equivalent to a single-pool Kt/Vurea value of 1.2 was initially established as a standard, which was incorporated into clinical practice guidelines and performance measures. The NCDS was not powered to evaluate mortality as an outcome. Although the NCDS was an exemplary early randomized, controlled trial of the adequacy of dialysis, the delivered dose in the low-dose treatment group was well below that routinely delivered today, and the patient population was not representative of current dialysis recipients.
In the 1990s, several large, observational studies suggested that doses of dialysis that were higher than standard doses and the use of dialysis membranes with higher-permeability characteristics (or flux) were associated with lower mortality.11-13,70-72 The Hemodialysis (HEMO) Study, funded by the National Institutes of Health, subsequently compared the effects of a standard dialysis dose (a single-pool Kt/Vurea value of 1.25) with a higher dose for urea clearance and also compared the effects of high versus low membrane flux on morbidity and mortality. A total of 1846 subjects were followed for 7 years, and the study had reasonably high power to detect a reduction in mortality. The results of the HEMO study showed no significant differences in all-cause mortality or in seven other prespecified outcomes among any of the treatment groups.73 Another well-conducted, randomized trial, the Adequacy of Peritoneal Dialysis in Mexico (ADEMEX) trial, also showed no relationship between dialysis dose and outcomes among patients receiving peritoneal dialysis.74Taken collectively, the results of these important trials suggest that there is a threshold–plateau relationship between the dose of dialysis and outcomes and that increasing the dose to greater than the currently recommended target of a single-pool Kt/Vurea value of 1.4 (in order to ensure an achieved dose of at least 1.2) does not improve important outcomes. These studies also illustrate the limitations of what is achievable with current dialysis practice and underscore the need for more innovative approaches.
CONTROLLED TRIALS TO EVALUATE CARDIOVASCULAR RISK
Many randomized, controlled trials have focused on mitigating cardiovascular events and mortality in patients undergoing hemodialysis, but results have been disappointing. Notably, two investigations evaluating the use of atorvastatin and rosuvastatin showed no improvement in major outcomes, despite being well powered because of the high cardiovascular event rate in each treatment group.58,59 Trials evaluating homocysteine-lowering drugs,75 non–calcium-containing phosphorus binders,76 and erythropoietin at doses that target higher hemoglobin concentrations77have all supported the null hypothesis or even suggested harm. In interpreting such trial results, it is important to consider that many metabolic and structural contributors to cardiovascular risk among patients undergoing dialysis may differ from those among patients without kidney disease (Figure 3)
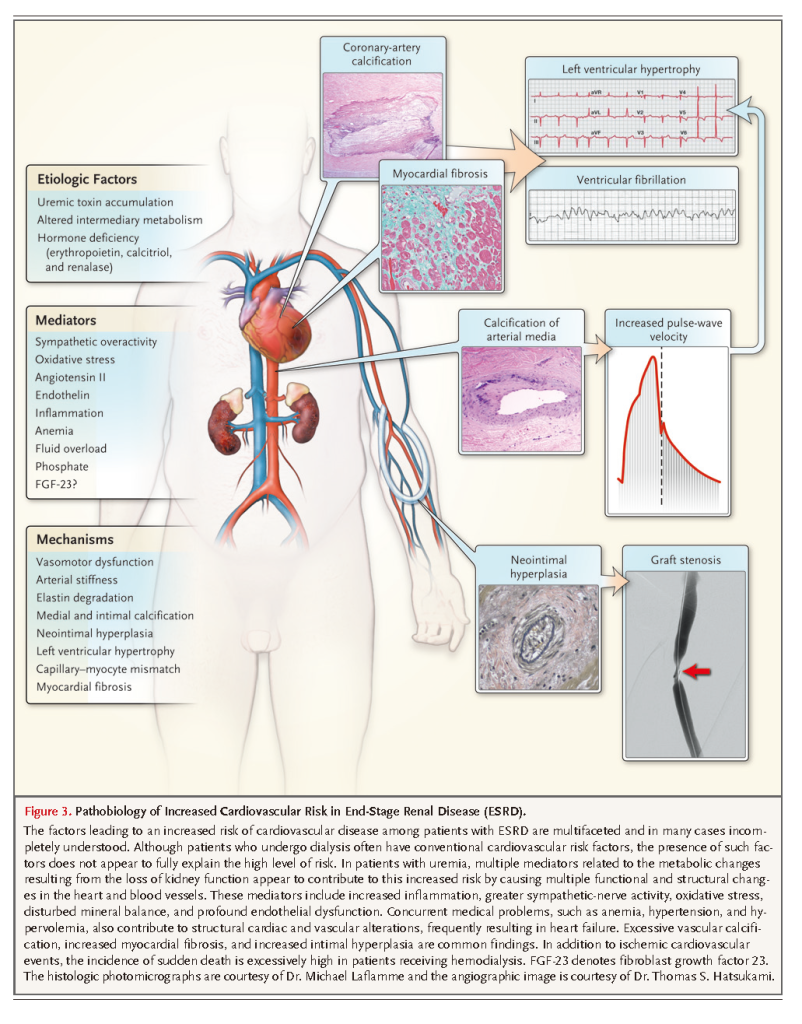
Furthermore, in cross-sectional studies, conventional cardiovascular risk factors, such as elevated serum cholesterol levels and blood pressure or a high degree of adiposity, have been found to be less predictive of risk among patients receiving dialysis than among persons with preserved kidney function.
Uremic cardiovascular disease is characterized by a high prevalence of medial vascular calcification, arterial stiffness, and altered left ventricular geometry.78-80 The development of aggressive intimal hyperplasia is common after either coronary angioplasty or the establishment of arteriovenous access.81 Cardiac arrest and congestive heart failure are more prominent causes of cardiovascular death than is acute myocardial infarction in patients with uremia.82 Metabolically, ESRD is strongly associated with acute inflammation, oxidative stress, endothelial dysfunction, insulin resistance, and excess sympathetic tone.83-91 A number of uremic toxins that are highly protein-bound or sequestered within cells or bone, such as p-cresol sulfate, indoxyl sulfate, and phosphate, may contribute directly to cardiovascular risk and are not sufficiently removed by means of conventional dialysis (Figure 1).5,10 Further research is needed to understand more precisely how uremic toxins contribute to cardiovascular risk and to evaluate novel approaches to reducing this risk. Innovative experimental approaches to dialysis have been advocated, such as wearable artificial kidneys and the use of nanotechnology for more rational membrane design.92-94 However, the large-scale implementation of any of these novel experimental approaches is not likely to occur in the near future.95
CONCLUSIONS
Over the past half century, the widespread use of dialysis to prolong life for people without kidney function has been a remarkable achievement. As a result of its growth and evolution, the U.S. ESRD Program has often provided an early window into social, political, and economic developments in health care, and these changes have later been reflected throughout the U.S. health care system. Despite such successes, the use of dialysis in the treatment of ESRD is problematic in some respects. The number of patients treated, especially in the United States, has escalated and is far beyond early estimates. Aggregate dialysis-associated costs have increased accordingly, and morbidity and mortality among treated patients remain high despite considerable technical and scientific improvements. Our knowledge of which uremic toxins confer injury and of how they can be optimally removed during dialysis therapy remains incomplete. The limited number of clinical trials that have attempted to improve outcomes have had disappointing results, so more well-designed and adequately powered clinical trials are needed.
Ongoing studies are assessing whether longer or more frequent dialysis treatments, or both, can improve outcomes and whether these changes would be acceptable to most patients. However, substantive improvements for patients receiving dialysis will probably require major technological breakthroughs that will be predicated on an improved understanding of uremic toxins and uremic complications.
REFERENCES
. 1 Scribner BH, Caner JE, Buri R, Quinton W. The technique of continuous hemodialysis. Trans Am Soc Artif Intern Organs 1960;6:88-103 Medline
. 2 Quinton W, Dillard D, Scribner BH. Cannulation of blood vessels for prolonged hemodialysis. Trans Am Soc Artif Intern Organs 1960;6:104-113 Medline
. 3 Depner TA. Prescribing hemodialysis: a guide to urea modeling. Boston: Kluwer Academic, 1991.
. 4 Locatelli F, Manzoni C, Di Filippo S. The importance of convective transport. Kidney Int Suppl 2002;115-20.
. 5 Meyer TW, Hostetter TH. Uremia. N Engl J Med 2007;357:1316-1325 Full Text | Web of Science | Medline
. 6 Gotch FA, Sargent JA. A mechanistic analysis of the National Cooperative Dialysis Study (NCDS). Kidney Int 1985;28:526-534 CrossRef | Web of Science | Medline
. 7 Daugirdas JT, Depner TA, Greene T, et al. Surface-area-normalized Kt/V: a method of rescaling dialysis dose to body surface area -- implications for different-size patients by gender. Semin Dial 2008;21:415-421 CrossRef | Web of Science | Medline
. 8 Port FK, Wolfe RA, Hulbert-Shearon TE, McCullough KP, Ashby VB, Held PJ. High dialysis dose is associated with lower mortality among women but not among men. Am J Kidney Dis 2004;43:1014-1023 CrossRef | Web of Science | Medline
. 9 Spalding EM, Chandna SM, Davenport A, Farrington K. Kt/V underestimates the hemodialysis dose in women and small men. Kidney Int 2008;74:348-355 CrossRef | Web of Science | Medline
. 10 Vanholder R, Baurmeister U, Brunet P, Cohen G, Glorieux G, Jankowski J. A bench to bedside view of uremic toxins. J Am Soc Nephrol 2008;19:863-870 CrossRef | Web of Science | Medline
. 11 Hornberger JC, Chernew M, Petersen J, Garber AM. A multivariate analysis of mortality and hospital admissions with high-flux dialysis. J Am Soc Nephrol 1992;3:1227-1237 Web of Science | Medline
. 12 Koda Y, Nishi S, Miyazaki S, et al. Switch from conventional to high-flux membrane reduces the risk of carpal tunnel syndrome and mortality of hemodialysis patients. Kidney Int 1997;52:1096-1101 CrossRef | Web of Science | Medline
. 13 Locatelli F, Mastrangelo F, Redaelli B, et al. Effects of different membranes and dialysis technologies on patient treatment tolerance and nutritional parameters. Kidney Int 1996;50:1293-1302 CrossRef | Web of Science | Medline
. 14 Port FK, Wolfe RA, Hulbert-Shearon TE, et al. Mortality risk by hemodialyzer reuse practice and dialyzer membrane characteristics: results from the USRDS dialysis morbidity and mortality study. Am J Kidney Dis 2001;37:276-286 CrossRef | Web of Science | Medline
. 15 Held PJ, Levin NW, Bovbjerg RR, Pauly MV, Diamond LH. Mortality and duration of hemodialysis treatment. JAMA 1991;265:871-875 CrossRef | Web of Science | Medline
. 16 Marshall MR, Byrne BG, Kerr PG, McDonald SP. Associations of hemodialysis dose and session length with mortality risk in Australian and New Zealand patients. Kidney Int 2006;69:1229-1236 CrossRef | Web of Science | Medline
. 17 Saran R, Bragg-Gresham JL, Levin NW, et al. Longer treatment time and slower ultrafiltration in hemodialysis: associations with reduced mortality in the DOPPS. Kidney Int 2006;69:1222-1228 CrossRef | Web of Science | Medline
. 18 Kalantar-Zadeh K, Regidor DL, Kovesdy CP, et al. Fluid retention is associated with cardiovascular mortality in patients undergoing long-term hemodialysis. Circulation 2009;119:671-679 CrossRef | Web of Science | Medline
. 19 Charra B, Calemard M, Laurent G. Importance of treatment time and blood pressure control in achieving long-term survival on dialysis. Am J Nephrol 1996;16:35-44 CrossRef | Web of Science | Medline
. 20 Powell JR, Oluwaseun O, Woo YM, et al. Ten years experience of in-center thrice weekly long overnight hemodialysis. Clin J Am Soc Nephrol 2009;4:1097-1101 CrossRef | Web of Science | Medline
. 21 Bugeja A, Dacouris N, Thomas A, et al. In-center nocturnal hemodialysis: another option in the management of chronic kidney disease. Clin J Am Soc Nephrol 2009;4:778-783 CrossRef | Web of Science | Medline
. 22 Troidle L, Hotchkiss M, Finkelstein F. A thrice weekly in-center nocturnal hemodialysis program. Adv Chronic Kidney Dis 2007;14:244-248 CrossRef | Web of Science | Medline
. 23 Kliger AS. High-frequency hemodialysis: rationale for randomized clinical trials. Clin J Am Soc Nephrol 2007;2:390-392 CrossRef | Web of Science | Medline
. 24 Suri RS, Nesrallah GE, Mainra R, et al. Daily hemodialysis: a systematic review. Clin J Am Soc Nephrol 2006;1:33-42 CrossRef | Web of Science | Medline
. 25 Culleton BF, Walsh M, Klarenbach SW, et al. Effect of frequent nocturnal hemodialysis vs conventional hemodialysis on left ventricular mass and quality of life: a randomized controlled trial. JAMA 2007;298:1291-1299 CrossRef | Web of Science | Medline
. 26 Suri RS, Garg AX, Chertow GM, et al. Frequent Hemodialysis Network (FHN) randomized trials: study design. Kidney Int 2007;71:349-359 CrossRef | Web of Science | Medline
. 27 Schreiner GE. How end-stage renal disease (ESRD)-Medicare developed. Am J Kidney Dis 2000;35:Suppl 1:S37-S44 CrossRef | Web of Science | Medline
. 28 Rettig RA. The policy debate on patient care financing for victims of end-stage renal disease. Law Contemp Probl 1976;40:196-230 CrossRef | Medline
. 29 Himmelfarb J, Berns A, Szczech L, Wesson D. Cost, quality, and value: the changing political economy of dialysis care. J Am Soc Nephrol 2007;18:2021-2027 CrossRef | Web of Science | Medline
. 30 Knauf F, Aronson PS. ESRD as a window into America's cost crisis in health care. J Am Soc Nephrol 2009;20:2093-2097 CrossRef | Web of Science | Medline
. 31 Rosansky SJ, Clark WF, Eggers P, Glassock RJ. Initiation of dialysis at higher GFRs: is the apparent rising tide of early dialysis harmful or helpful? Kidney Int 2009;76:257-261 CrossRef | Web of Science | Medline
. 32 Kurella Tamura M, Covinsky KE, Chertow GM, Yaffe K, Landefeld CS, McCulloch CE. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med 2009;361:1539-1547 Full Text | Medline
. 33 Arnold RM, Zeidel ML. Dialysis in frail elders -- a role for palliative care. N Engl J Med 2009;361:1597-1598 Full Text | Web of Science | Medline
. 34 Moss AH. Shared decision-making in dialysis: the new RPA/ASN guideline on appropriate initiation and withdrawal of treatment. Am J Kidney Dis 2001;37:1081-1091 CrossRef | Web of Science | Medline
. 35 Murtagh FE, Marsh JE, Donohoe P, Ekbal NJ, Sheerin NS, Harris FE. Dialysis or not? A comparative survival study of patients over 75 years with chronic kidney disease stage 5. Nephrol Dial Transplant 2007;22:1955-1962 CrossRef | Web of Science | Medline
. 36 Himmelfarb J, Kliger AS. End-stage renal disease measures of quality. Annu Rev Med 2007;58:387-399 CrossRef | Web of Science | Medline
. 37 Kidney Disease: Improving Global Outcomes (KDIGO) home page. (http://kdigo.org/.)
. 38 National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF KDOQI). New York: National Kidney Foundation. (http://www.kidney.org/professionals/KDOQI/.)
. 39 National AV fistula rate reaches 55.3% in April 2010. Midlothian, VA: Fistula First Breakthrough Initiative. (http://www.fistulafirst.org.)
. 40 Dialysis Facility Compare (DFC). Baltimore: Centers for Medicare & Medicaid Services. (http://www.cms.gov/dialysisfacilitycompare/.)
. 41 Oliver MJ, Edwards LJ, Churchill DN. Impact of sodium and ultrafiltration profiling on hemodialysis-related symptoms. J Am Soc Nephrol 2001;12:151-156 Web of Science | Medline
. 42 Lopot F, Nejedly B, Sulkova S, Blaha J. Comparison of different techniques of hemodialysis vascular access flow evaluation. J Vasc Access 2004;5:25-32 Medline
. 43 Maduell F, Vera M, Arias M, et al. Influence of the ionic dialysance monitor on Kt measurement in hemodialysis. Am J Kidney Dis 2008;52:85-92 CrossRef | Web of Science | Medline
. 44 Ward RA, Ronco C. Improvements in technology: a path to safer and more effective hemodialysis. Blood Purif 2009;27:6-10 CrossRef | Web of Science | Medline
. 45 Maggiore Q, Pizzarelli F, Santoro A, et al. The effects of control of thermal balance on vascular stability in hemodialysis patients: results of the European randomized clinical trial. Am J Kidney Dis 2002;40:280-290 CrossRef | Web of Science | Medline
. 46 Locatelli F, Buoncristiani U, Canaud B, Kohler H, Petitclerc T, Zucchelli P. Haemodialysis with on-line monitoring equipment: tools or toys? Nephrol Dial Transplant 2005;20:22-33 CrossRef | Web of Science | Medline
. 47 US Renal Data System. USRDS 2009 annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States. 2009. (http://www.usrds.org/adr.htm.)
. 48 Lowrie EG, Lew NL. Death risk in hemodialysis patients: the predictive value of commonly measured variables and an evaluation of death rate differences between facilities. Am J Kidney Dis 1990;15:458-482 Web of Science | Medline
. 49 Ward RA. Ultrapure dialysate. Semin Dial 2004;17:489-497 CrossRef | Web of Science | Medline
. 50 Pfeffer MA, Burdmann EA, Chen CY, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 2009;361:2019-2032 Full Text | Web of Science | Medline
. 51 Singh AK, Szczech L, Tang KL, et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 2006;355:2085-2098 Full Text | Web of Science | Medline
. 52 Drueke TB, Locatelli F, Clyne N, et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 2006;355:2071-2084 Full Text | Web of Science | Medline
. 53 Unger EF, Thompson AM, Blank MJ, Temple R. Erythropoiesis-stimulating agents -- time for a reevaluation. N Engl J Med 2010;362:189-192 Full Text | Web of Science | Medline
. 54 Spiegel DM, Chertow GM. Lost without directions: lessons from the anemia debate and the DRIVE study. Clin J Am Soc Nephrol 2009;4:1009-1010 CrossRef | Web of Science | Medline
. 55 Lacson E, Lazarus JM, Himmelfarb J, Ikizler TA, Hakim RM. Balancing Fistula First with Catheters Last. Am J Kidney Dis 2007;50:379-395 CrossRef | Web of Science | Medline
. 56 Center for Medicaid and State Operations/Survey & Certification Group. End-stage renal disease (ESRD) program interpretive guidance, version 1.1. Baltimore: Centers for Medicare & Medicaid Services, 2008. (http://www.cms.gov/SurveyCertificationGenInfo/downloads/SCletter09-01.pdf.)
. 57 Agarwal R. Assessment of blood pressure in hemodialysis patients. Semin Dial 2002;15:299-304 CrossRef | Web of Science | Medline
. 58 Fellstrom BC, Jardine AG, Schmeider RE, et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med 2009;360:1395-1407[Erratum, N Engl J Med 2010;362:1450.] Full Text | Web of Science | Medline
. 59 Wanner C, Krane V, Marz W, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med 2005;353:238-248[Erratum, N Engl J Med 2005;353:1640.] Full Text | Web of Science | Medline
. 60 Liu Y, Coresh J, Eustace JA, et al. Association between cholesterol level and mortality in dialysis patients: role of inflammation and malnutrition. JAMA 2004;291:451-459 CrossRef | Web of Science | Medline
. 61 Williams ME, Lacson E, Teng M, Ofsthun N, Lazarus JM. Hemodialyzed type I and type II diabetic patients in the US: characteristics, glycemic control, and survival. Kidney Int 2006;70:1503-1509 CrossRef | Web of Science | Medline
. 62 Lowrie EG, Curtin RB, LePain N, Schatell D. Medical Outcomes Study Short Form-36: a consistent and powerful predictor of morbidity and mortality in dialysis patients. Am J Kidney Dis 2003;41:1286-1292 CrossRef | Web of Science | Medline
. 63 Himmelfarb J, Chuang P, Schulman G. Hemodialysis. 8th ed. Philadelphia: W.B. Saunders, 2008.
. 64 Young EW, Goodkin DA, Mapes DL, et al. The Dialysis Outcomes and Practice Patterns Study (DOPPS): an international hemodialysis study. Kidney Int 2000;57:Suppl:S74-S81 CrossRef | Web of Science
. 65 Goodkin DA, Young EW, Kurokawa K, Prutz KG, Levin NW. Mortality among hemodialysis patients in Europe, Japan, and the United States: case-mix effects. Am J Kidney Dis 2004;44:16-21 CrossRef | Medline
. 66 Goodkin DA, Bragg-Gresham JL, Koenig KG, et al. Association of comorbid conditions and mortality in hemodialysis patients in Europe, Japan, and the United States: the Dialysis Outcomes and Practice Patterns Study (DOPPS). J Am Soc Nephrol 2003;14:3270-3277 CrossRef | Web of Science | Medline
. 67 Foley RN, Hakim RM. Why is the mortality of dialysis patients in the United States much higher than the rest of the world? J Am Soc Nephrol 2009;20:1432-1435 CrossRef | Web of Science | Medline
. 68 Yoshino M, Kuhlmann MK, Kotanko P, et al. International differences in dialysis mortality reflect background general population atherosclerotic cardiovascular mortality. J Am Soc Nephrol 2006;17:3510-3519 CrossRef | Web of Science | Medline
. 69 Lowrie EG, Laird NM, Parker TF, Sargent JA. Effect of the hemodialysis prescription of patient morbidity: report from the National Cooperative Dialysis Study. N Engl J Med 1981;305:1176-1181 Full Text | Web of Science | Medline
. 70 Collins AJ, Ma JZ, Umen A, Keshaviah P. Urea index and other predictors of hemodialysis patient survival. Am J Kidney Dis 1994;23:272-282 Web of Science | Medline
. 71 Held PJ, Port FK, Wolfe RA, et al. The dose of hemodialysis and patient mortality. Kidney Int 1996;50:550-556 CrossRef | Web of Science | Medline
. 72 Owen WF, Lew NL, Liu Y, Lowrie EG, Lazarus JM. The urea reduction ratio and serum albumin concentration as predictors of mortality in patients undergoing hemodialysis. N Engl J Med 1993;329:1001-1006 Full Text | Web of Science | Medline
. 73 Eknoyan G, Beck GJ, Cheung AK, et al. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med 2002;347:2010-2019 Full Text | Web of Science | Medline
. 74 Paniagua R, Amato D, Vonesh E, et al. Effects of increased peritoneal clearances on mortality rates in peritoneal dialysis: ADEMEX, a prospective, randomized, controlled trial. J Am Soc Nephrol 2002;13:1307-1320 Medline
. 75 Jamison RL, Hartigan P, Kaufman JS, et al. Effect of homocysteine lowering on mortality and vascular disease in advanced chronic kidney disease and end-stage renal disease: a randomized controlled trial. JAMA 2007;298:1163-1170[Erratum, JAMA 2008;300:170.] CrossRef | Web of Science | Medline
. 76 Suki WN, Zabaneh R, Cangiano JL, et al. Effects of sevelamer and calcium-based phosphate binders on mortality in hemodialysis patients. Kidney Int 2007;72:1130-1137 CrossRef | Web of Science | Medline
. 77 Besarab A, Bolton WK, Browne JK, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med 1998;339:584-590 Full Text | Web of Science | Medline
. 78 Middleton RJ, Parfrey PS, Foley RN. Left ventricular hypertrophy in the renal patient. J Am Soc Nephrol 2001;12:1079-1084 Web of Science | Medline
. 79 Block GA, Raggi P, Bellasi A, Kooienga L, Spiegel DM. Mortality effect of coronary calcification and phosphate binder choice in incident hemodialysis patients. Kidney Int 2007;71:438-441 CrossRef | Web of Science | Medline
. 80 Raggi P, Bellasi A, Ferramosca E, Islam T, Muntner P, Block GA. Association of pulse wave velocity with vascular and valvular calcification in hemodialysis patients. Kidney Int 2007;71:802-807 CrossRef | Web of Science | Medline
. 81 Li L, Terry CM, Shiu YT, Cheung AK. Neointimal hyperplasia associated with synthetic hemodialysis grafts. Kidney Int 2008;74:1247-1261 CrossRef | Web of Science | Medline
. 82 Herzog CA, Mangrum JM, Passman R. Sudden cardiac death and dialysis patients. Semin Dial 2008;21:300-307 CrossRef | Web of Science | Medline
. 83 Converse RL, Jacobsen TN, Toto RD, et al. Sympathetic overactivity in patients with chronic renal failure. N Engl J Med 1992;327:1912-1918 Full Text | Web of Science | Medline
. 84 Annuk M, Zilmer M, Lind L, Linde T, Fellstrom B. Oxidative stress and endothelial function in chronic renal failure. J Am Soc Nephrol 2001;12:2747-2752 Web of Science | Medline
. 85 Zoccali C, Mallamaci F, Tripepi G, et al. Adiponectin, metabolic risk factors, and cardiovascular events among patients with end-stage renal disease. J Am Soc Nephrol 2002;13:134-141 CrossRef | Web of Science | Medline
. 86 DeFronzo RA, Alvestrand A, Smith D, Hendler R, Hendler E, Wahren J. Insulin resistance in uremia. J Clin Invest 1981;67:563-568 CrossRef | Web of Science | Medline
. 87 Himmelfarb J, Stenvinkel P, Ikizler TA, Hakim RM. The elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int 2002;62:1524-1538 CrossRef | Web of Science | Medline
. 88 Ikizler TA, Wingard RL, Harvell J, Shyr Y, Hakim RM. Association of morbidity with markers of nutrition and inflammation in chronic hemodialysis patients: a prospective study. Kidney Int 1999;55:1945-1951 CrossRef | Web of Science | Medline
. 89 Yilmaz MI, Carrero JJ, Ortiz A, et al. Soluble TWEAK plasma levels as a novel biomarker of endothelial function in patients with chronic kidney disease. Clin J Am Soc Nephrol 2009;4:1716-1723 CrossRef | Web of Science | Medline
. 90 Vallance P, Leone A, Calver A, Collier J, Moncada S. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet 1992;339:572-575 CrossRef | Web of Science | Medline
. 91 Passauer J, Bussemaker E, Range U, Plug M, Gross P. Evidence in vivo showing increase of baseline nitric oxide generation and impairment of endothelium-dependent vasodilation in normotensive patients on chronic hemodialysis. J Am Soc Nephrol 2000;11:1726-1734 Web of Science | Medline
. 92 Fissell WH, Roy S. The implantable artificial kidney. Semin Dial 2009;22:665-670 CrossRef | Web of Science | Medline
. 93 Gura V, Macy AS, Beizai M, Ezon C, Golper TA. Technical breakthroughs in the wearable artificial kidney (WAK). Clin J Am Soc Nephrol 2009;4:1441-1448 CrossRef | Web of Science | Medline
. 94 Nissenson AR, Ronco C, Pergamit G, Edelstein M, Watts R. Continuously functioning artificial nephron system: the promise of nanotechnology. Hemodial Int 2005;9:210-217 CrossRef | Medline
. 95 Rastogi A, Nissenson AR. Technological advances in renal replacement therapy: five years and beyond. Clin J Am Soc Nephrol 2009;4:Suppl 1:S132-S136 CrossRef | Web of Science | Medline





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 