Betty Y.S. Kim, M.D., Ph.D., James T. Rutka, M.D., Ph.D., and Warren C.W. Chan, Ph.D.
N Engl J Med 2010; 363:2434-2443
Many diseases originate from alterations in biologic processes at the molecular or nanoscale level. Mutated genes, misfolded proteins, and infections caused by viruses or bacteria can lead to cell malfunction or miscommunication, sometimes leading to life-threatening diseases. These molecules and infectious agents are nanometers in size and may be located in biologic systems that are protected by nanometer-size barriers, such as nuclear pores 9 nm in diameter. Their chemical properties, size, and shape appear to dictate the transport of molecules to specific biologic compartments and the interactions between molecules.
Nanotechnology is defined as the “intentional design, characterization, production, and applications of materials, structures, devices, and systems by controlling their size and shape in the nanoscale range (1 to 100 nm).”1 Because nanomaterials are similar in scale to biologic molecules and systems yet can be engineered to have various functions, nanotechnology is potentially useful for medical applications. The field of nanomedicine aims to use the properties and physical characteristics of nanomaterials for the diagnosis and treatment of diseases at the molecular level.
Nanomaterials are now being designed to aid the transport of diagnostic or therapeutic agents through biologic barriers; to gain access to molecules; to mediate molecular interactions; and to detect molecular changes in a sensitive, high-throughput manner. In contrast to atoms and macroscopic materials, nanomaterials have a high ratio of surface area to volume as well as tunable optical, electronic, magnetic, and biologic properties, and they can be engineered to have different sizes, shapes, chemical compositions, surface chemical characteristics, and hollow or solid structures.2,3 These properties are being incorporated into new generations of drug-delivery vehicles, contrast agents, and diagnostic devices, some of which are currently undergoing clinical investigation or have been approved by the Food and Drug Administration (FDA) for use in humans. Examples of the nanomaterials most commonly used in medicine are provided in Figure 1. and table1
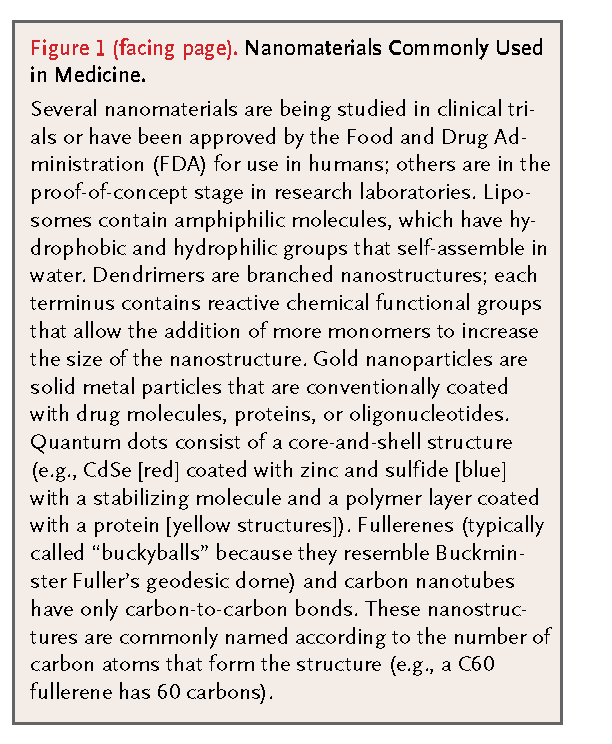
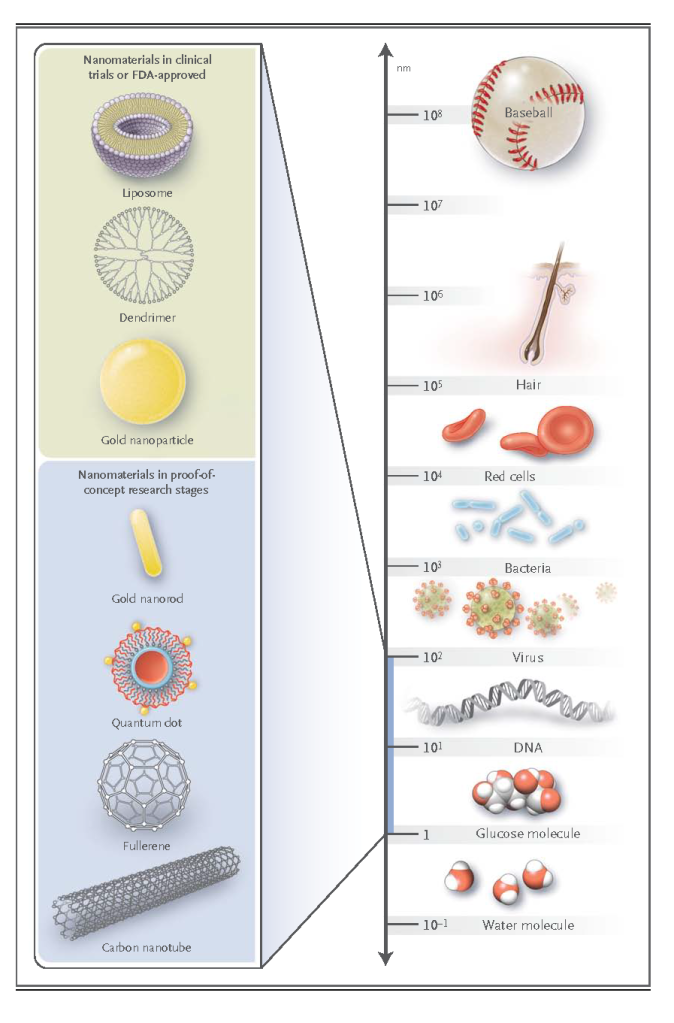
This overview describes the properties of nanomaterials, their principal medical applications, and the future possibilities for this emerging field.
PROPERTIES OF NANOMATERIALS
Over the past three decades, physical scientists have developed strategies to reproducibly synthesize nanomaterials and to characterize their unique, size-dependent properties.2,3 An understanding of these fundamental physical and chemical properties is necessary for the optimal use of nanomaterials in medical applications.
Nanomaterials generally consist of metal atoms, nonmetal atoms, or a mixture of metal and nonmetal atoms, commonly referred to as metallic, organic, or semiconducting particles, respectively. The surface of nanomaterials is usually coated with polymers or biorecognition molecules for improved biocompatibility and selective targeting of biologic molecules. The final size and structure of nanomaterials depend on the salt and surfactant additives, reactant concentrations, reaction temperatures, and solvent conditions used during their synthesis.
A common feature of all nanomaterials is their large ratio of surface area to volume, which may be orders of magnitude greater than that of macroscopic materials.4 Cutting a 1-cm cube into 1021 cubes that are each 1 nm on a side will result in the same overall volume and mass, but the surface area will be increased by a factor of 10 million. Thus, the advantage of using nanomaterials as carriers is that their surface can be coated with many molecules.
Unique aspects of metal-containing materials with at least one dimension that is smaller than 100 nm are their size, shape, and composition-tunable electronic, magnetic, and optical properties. This relationship is a direct consequence of the behavior of electrons in the nanomaterial. Electrons have two important characteristics: their spin and their ability to move in a quantized fashion between specific energy levels. Electrons are similar to tiny bar magnets, with a surrounding magnetic field that corresponds to the electron spin in an applied field. Also, after absorbing energy, electrons can generate light or heat when they move between different energy levels (Figure 2)
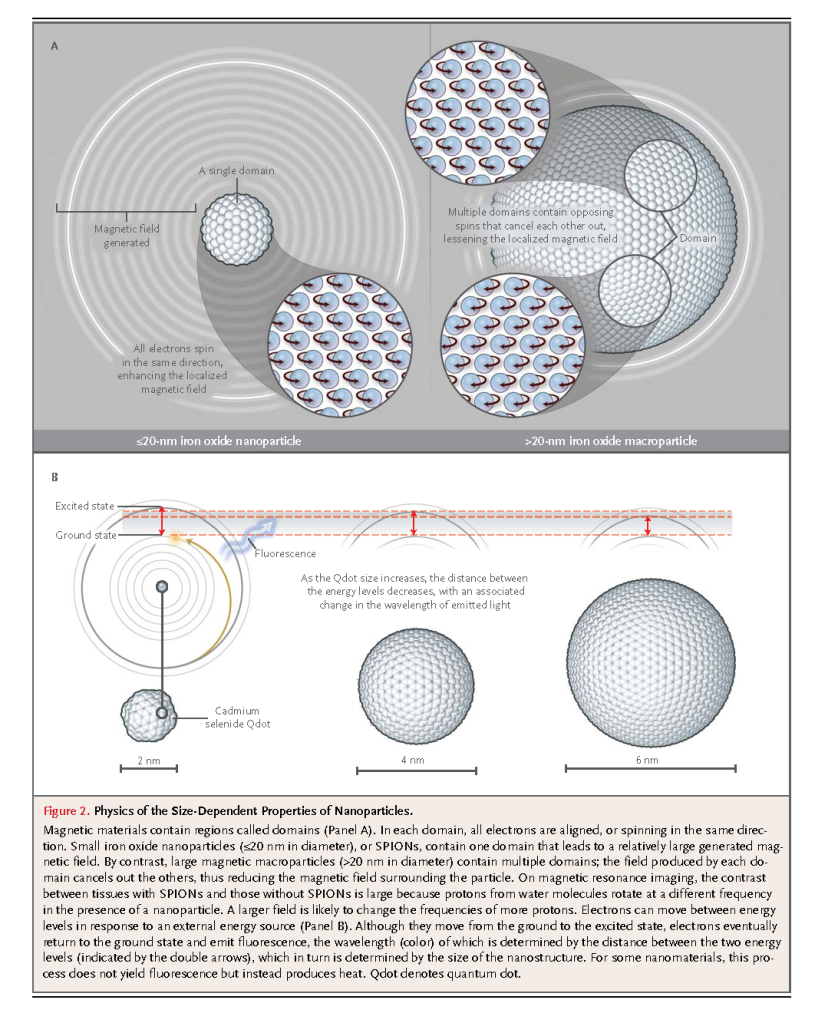
In macrostructures, electrons can spin in two directions, in opposition or in alignment, and can move among many energy levels. The behavior of electrons in nanostructures is more constrained and depends on the size or shape of the material or on the electrons' interactions with the surface coating. The chemical composition of a nanomaterial determines whether one or both electron characteristics (spin and energy transition) are affected, as well as the extent of that effect. For example, all electrons in iron oxide magnetic nanoparticles (≤20 nm in diameter) spin in the same direction, whereas electrons in iron oxide macroparticles (>20 nm in diameter) spin in opposite directions (Figure 2A).5 When these spins are aligned in the same direction, the field becomes additive, but when the electrons spin in opposite directions, the fields cancel each other out. Since the overall magnetic-field strength of a material is the sum of the magnetic fields of individual electrons, these nanoparticles have a larger, localized magnetic field as compared with that of larger particles. This larger magnetic field can increase the contrast on magnetic resonance imaging (MRI), since more protons interact in a larger field.
In cadmium selenide semiconductor nanostructures that are less than 10 nm in diameter (known as CdSe quantum dots, or Qdots), the electrons can transition between two energy levels: a ground state, in which the electrons are at rest, and an excited state, in which they are mobile (Figure 2B).6The difference between the ground and excited energy levels dictates the color and fluorescence emission of these nanostructures. This energy difference is size-dependent and can be observed under ultraviolet light by means of the fluorescence emissions of Qdots of different sizes. Furthermore, the magnetic and optical signals from these inorganic nanomaterials tend to be stronger than their traditional molecular counterparts because a larger number of electrons are involved. To illustrate this point, the absorption cross section of Qdots, a measure of the number of electrons that transition from the ground to the excited state, is at least 10 times that of organic fluorescent dye molecules.6
NANOMATERIALS FOR IN VIVO APPLICATIONS
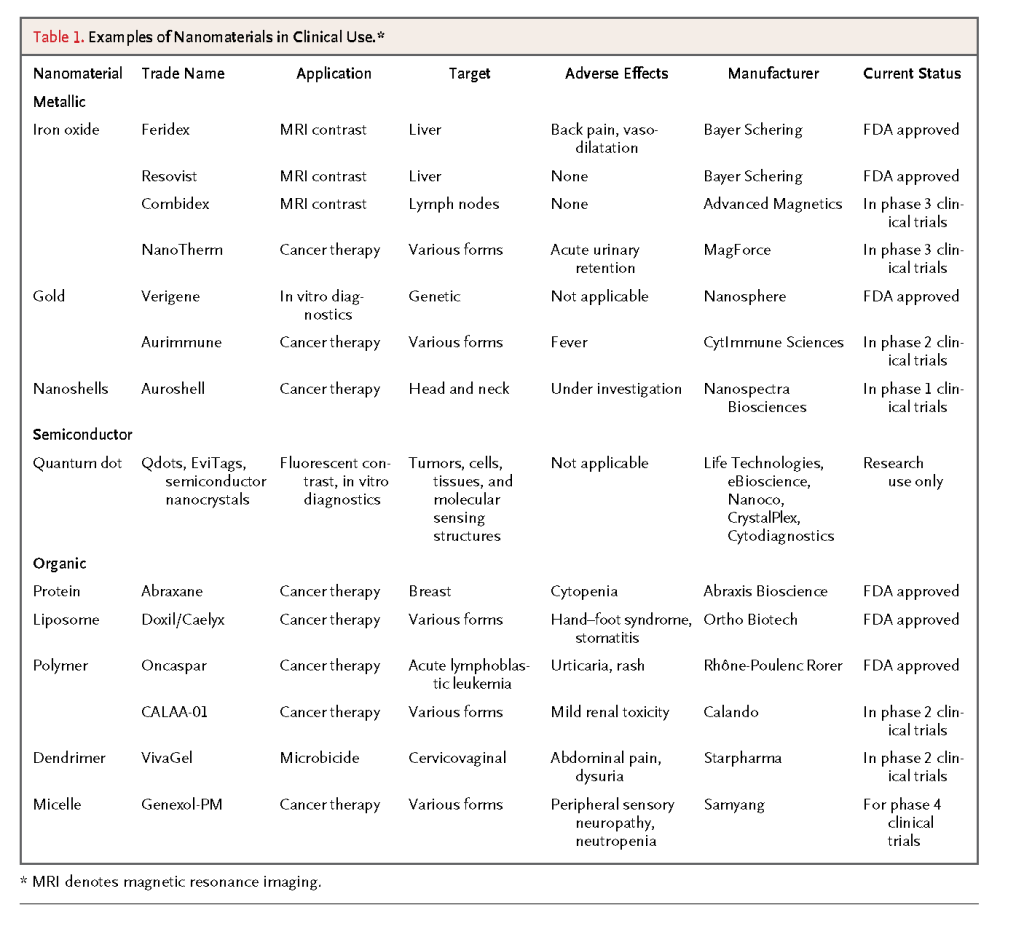
A handful of nanomaterials are being studied in clinical trials or have already been approved by the FDA for use in humans,3,7,8 and many proof-of-concept studies of nanomaterials in cell-culture and small-animal models for medical applications are under way.6,9,10 Many of these nanomaterials are designed to target tumors in vivo and are intended for use either as drug carriers for therapeutic applications or as contrast agents for diagnostic imaging (Figure3)
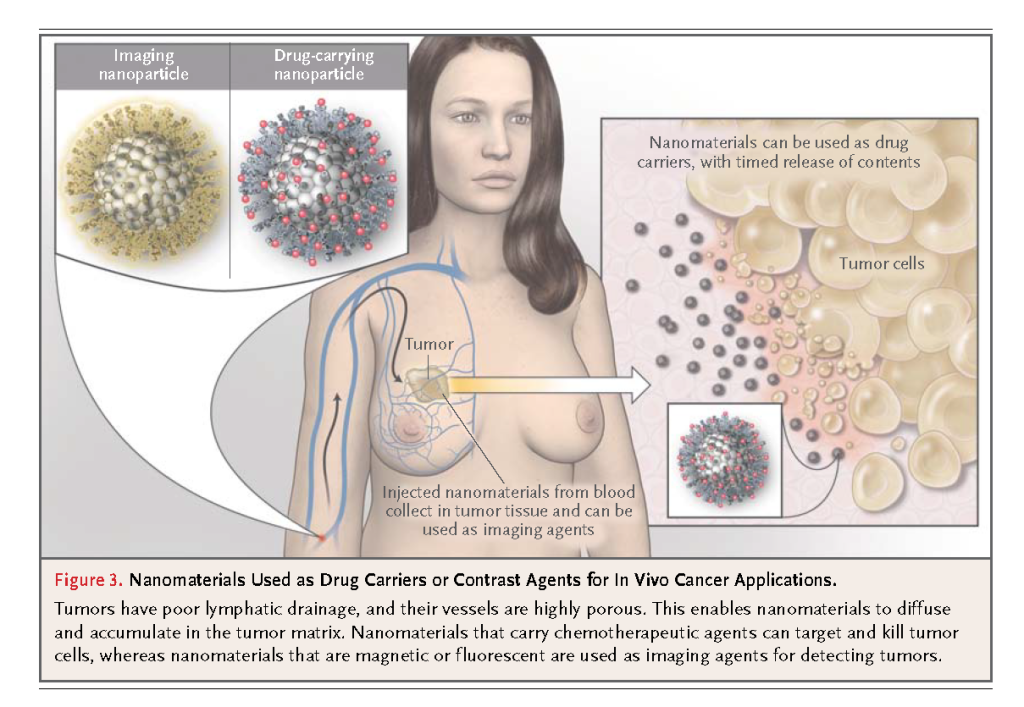
Nanomaterials infused into the bloodstream can accumulate in tumors owing to the enhanced permeability and retention effect when the vasculature of immature tumors has pores smaller than 200 nm, permitting extravasation of nanoparticles from blood into tumor tissue. 11 The infusion of antineoplastic drugs with nanomaterials as carriers results in an increased payload of drugs to the tumor, as compared with conventional infusion. With nanomaterials, the high ratio of surface area to volume permits high surface loading of therapeutic agents; in the case of organic nanomaterials, their hollow or porous core allows encapsulation of hundreds of drug molecules within a single carrier particle. When the carrier particle degrades, the drug molecules are released, and the rate of degradation can even be controlled and fine-tuned according to the polymer composition. These nanomaterial delivery vehicles can also be coated with polymers, such as polyethylene glycol, to increase their half-life in the blood circulation, prevent opsonizing proteins from adhering to the nanomaterial surface, and reduce rapid metabolism and clearance. Moreover, the use of nanomaterials for drug delivery may minimize adverse effects by preventing the nonspecific uptake of therapeutic agents into healthy tissues.12,13
Aurimmune (CytImmune Sciences), which consists of 27-nm gold nanoparticles coated with recombinant human tumor necrosis factor alpha (TNF-α) and polyethylene glycol, is under investigation in phase 2 clinical trials (ClinicalTrials.gov numbers, NCT00356980 and NCT00436410) for the treatment of patients with a variety of advanced or metastatic cancers who are no longer responsive to conventional treatment. Histopathological studies have shown that these nanoparticles are localized within or around the tumor, with less uptake into healthy organs than is seen with the direct injection of TNF-α. The toxic effects and nonspecific accumulation normally associated with direct injection of TNF-α are reduced when TNF-α is coated on the nanoparticle surface. The use of cytokines such as TNF-α is limited by the inflammatory responses they produce, especially when tissues are exposed to high doses. With intravenous injection of Aurimmune, patients are able to tolerate 20 times the usual dose of TNF-α.14,15
Another agent under investigation and now in a phase 4 clinical trial (NCT00912639) is Genexol-PM (Samyang), which consists of 20-nm to 50-nm micelles formed by the self-assembly of polyethylene glycol and poly-D,L-lactide polymers. The core of these micelles contains paclitaxel, the chemotherapeutic mitotic inhibitor. The micelles were injected into 21 patients with advanced solid tumors that were refractory to conventional therapies. The disease stabilized in 42% of patients, and in 14% of patients, there were positive responses (e.g., a decrease in lung mass).16In both these examples, the patients were able to tolerate a higher drug dose owing to the altered pharmacokinetic behavior of the therapeutic agents with the use of nanoparticles, with no apparent side effects attributable to the nanoparticle carrier.
Nanoparticles are also attractive as sensitive contrast agents for cancer imaging. On nanoparticle-enhanced MRI, a contrast can be observed between tissues with and those without superparamagnetic iron oxide nanoparticles (SPIONs), owing to a difference in the precession frequency of the protons (see the Supplementary Appendix, available with the full text of this article at NEJM.org). In one study, dextran-coated SPIONs were injected into patients with prostate cancer to detect possible lymph-node metastases.17 The dextran coating increased the circulation time of the nanoparticles, and because of their small size, these particles could traverse the lymphatic vessels to reach the lymph nodes and be taken up by the resident macrophages. The use of SPIONs with MRI, as compared with conventional MRI, was associated with substantial increases in both diagnostic sensitivity (90.5% vs. 35.4%) and specificity (97.9% vs. 90.4%) in the detection of metastatic tumors.
In another study, SPIONs were injected into patients with solid tumors. The SPIONs remained in the tumors 24 hours after the injection, as compared with 1 hour for gadolinium-chelate contrast agents.18 The reason for this difference is that the smaller nanoparticles are more easily taken up by tumor cells and diffuse out of the tumor more slowly.19 As a result, the tumor margins can be distinguished on MRI for a longer period. Magnetic nanoparticles are also being studied in clinical trials for imaging of hyperplasia, adenoma, and more specifically, primary lung cancer, in which a decrease in the function of the reticuloendothelial system affects the amount of nonspecific phagocytic uptake.
NANOMATERIALS FOR IN VITRO DIAGNOSIS
The second key application of nanomaterials is as a label for measuring molecules of interest in biologic samples. Nanomaterials are used to either simplify the readout or amplify the detection threshold of the diagnostic device. Nanoparticles are used in lateral-flow in vitro diagnostic assays (LFA) (as described below), such as the urine pregnancy test for detecting protein markers (e.g., human chorionic gonadotropin [hCG]).20 The hCG molecule is introduced into a membrane strip, which moves through the membrane by capillary force and initially interacts with anti-hCG antibody–coated gold nanoparticles. On successful binding, this complex moves through the membrane until it recognizes a region that is also coated with anti-hCG antibody. The complex becomes tethered to the membrane surface as a result of the antigen–antibody interaction. The accumulation of gold nanoparticles in this region of the membrane now appears red, which is deeper and richer in color than that of organic chromophores because the gold nanoparticles have a larger cross section of absorption. This allows an easier readout of the signal at the point of care without the need for a more complex instrument. A number of FDA-approved LFAs for measuring human immunodeficiency virus (HIV), malaria, and cardiac markers are also available. Although this technique is simple to use and can be carried out rapidly (in less than 1 hour), it suffers from poor detection thresholds (millimolar to micromolar, depending on the biomarker).
Gold nanoparticles are also used in high-throughput genomic detection devices without the need for polymerase-chain-reaction (PCR) amplification but with a sensitivity similar to that of PCR-based assays (Figure4)
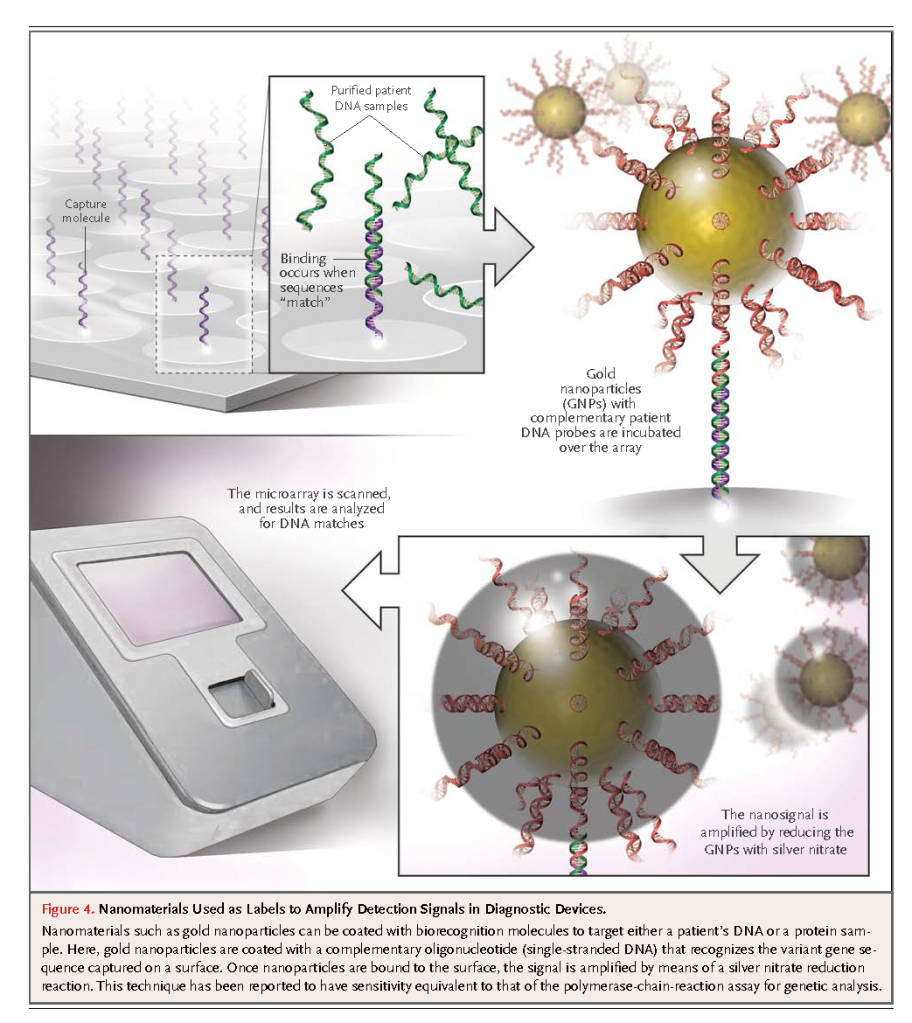
This technology has been approved by the FDA for genetic screening to determine drug sensitivity and to detect genetic mutations. One particular application is in screening for single-nucleotide polymorphisms of the factor V and factor II genes and the 5,10-methylenetetrahydrofolate reductase gene, in which the mutations are related to thrombophilia and hyperhomocysteinemia.22 A library of genetic sequences is coated onto a microarray surface. Isolated and purified genetic materials obtained from patient samples are introduced into the microarray, followed by incubation with gold-nanoparticle probes. Gene-specific oligonucleotides link the gold nanoparticles to the surface. Finally, the sample is washed to remove the unbound particles and is then amplified by reducing the remaining gold nanoparticles with the chemical agents silver nitrate and hydroquinone to produce a black spot on the microarray. This approach does not suffer from the problems often associated with conventional fluorescent probes for microarray labeling, such as photobleaching (loss of signal after exposure to light), and can detect multiple markers with a high sensitivity (95%) and low detection threshold (10−18 M). A modification of this approach called the bio-barcode assay is currently being validated for the detection of proteins found in prostate cancer.23
OTHER NANOMATERIAL-BASED CLINICAL APPLICATIONS
Nanomaterials are being put to other clinical uses. For example, gold nanoshells, which comprise a silica core coated with a thin layer of gold, are being used for the treatment of recurrent head and neck tumors. In this application, the nanoshells are injected into the tumor and then illuminated with 700-nm to 800-nm light.24 Electrons excited by these wavelengths interact with the surrounding water molecules, causing localized heating and subsequent cell death. Rod-shaped gold nanoparticles25 and carbon nanotubes can also produce heat on optical excitation, but to date their use has been limited to mouse models.
Nanomaterials are also being evaluated in clinical trials as agents for inhibiting the spread of sexually transmitted diseases (NCT00370357, NCT00442910, and NCT00740584). These nanomaterials are engineered to disrupt the biologic interactions between the pathogen and the host.7,26, Dendrimers, which are nanostructures that look like tree branches (Figure 1), have been shown to prevent the transmission of HIV in macaque models. The mechanism of inhibition depends on the dendrimer's surface chemical features and size. Dendrimers with benzene dicarboxylate on the surface inhibit HIV from entering the cell by binding to its viral capsid; conversely, dendrimers with naphthalene disulfonate on the surface enter the cells and inhibit reverse-transcriptase and integrase activity. These differential cellular-uptake mechanisms have not been fully elucidated and are under investigation.27 The two enzymes mentioned are involved in translating the RNA from HIV into DNA and integrating it into the host DNA. It has been proposed that the dendrimer size mimics the ligand, whereas its multivalency strengthens the interaction with the biologic target. In both cases, the therapeutic benefit persists because the virus cannot function normally and cannot be transmitted from one macaque to another.
CURRENT CHALLENGES AND FUTURE OUTLOOK
There is growing speculation about possible nanomaterial toxicity on the basis of in vitro cell-culture studies and in vivo animal studies. For example, the metabolism of CdSe Qdots leads to cadmium toxicity, with adverse effects on the function, viability, and morphologic features of primary (freshly isolated) rat hepatocytes.28 Carbon nanotubes can induce asbestos-like inflammation and granulomas in female mice.29 It is possible that the heavy-metal composition and physical properties of nanomaterials, as well as their ability to enter vital organs, lead to unique toxic responses. To date, there is no conclusive evidence of a known human toxic response that is specifically caused by nanomaterials. However, a study showed that high doses of nanoparticle-based therapeutic agents (27 mg of small inhibitory RNA [siRNA] vs. 9 mg per kilogram in 40-nm nanoparticles) led to reversible kidney toxicity.30 Magnetic nanoparticles used for thermal ablation have been shown to be retained in the urinary tract (in patients with a history of urethral stricture) and to result in treatment-related illness.31 In contrast, numerous studies have shown that certain nanomaterials do not elicit toxic responses in animals, as determined by histopathological studies and analyses of hepatic and renal markers.32,33 The issue of nanomaterial toxicity remains controversial and requires more study.
Nanomaterials are frequently used in basic research as probes for studying the molecular basis of diseases or in proof-of-concept studies performed to demonstrate their medical usefulness. For example, fluorescence microscopy revealed the binding and transport of Qdots coated with epidermal growth factor to ErbB/Her receptors overexpressed in breast and ovarian cancer, thereby allowing researchers to identify the specific receptor subtype responsible for this molecular interaction.34 Nanomaterials are being explored for many applications, including cell and tissue screening35 and in vivo targeting of tumors with the use of nanoparticles coated with antibodies, peptides, or oligonucleotides folded into complex structures called aptamers.36,37 In addition, nanomaterials are being used as platforms for designing multifunctional agents with diagnostic and therapeutic capabilities (silicon nanostructures that contain the DNA-intercalating drug doxorubicin with luminescence properties for imaging)38,39; and as antioxidants (fullerenes, or “buckyballs,” which contain about 30 double bonds that react with free radicals in tissues).40 To translate these applications into clinical use, researchers must optimize the nanomaterials, beginning with small-animal models and scaling up to nonhuman primate models — a process that will take some time. These studies should provide a solid foundation for the long-term advancement of nanotechnology into an effective new area in clinical medical practice.
Supported by grants from the University of Toronto Department of Surgery Surgeon Scientist Program and Clinical Investigator Program, the Neurosurgery Research and Education Foundation–American Association of Neurological Surgeons (to Dr. Kim), the Natural Sciences and Engineering Research Council of Canada (to Dr. Kim and RGPIN288231-09 and NETGP35015-07 to Dr. Chan), the Canadian Institute of Health Research (MOP-74610, to Dr. Rutka; and RMF-72551 and MOP-93532 to Dr. Chan), the Canadian Cancer Society Research Institute (to Dr. Rutka), and the Canadian Foundation for Innovation and the Ontario Innovation Trust (to Dr. Chan).
Dr. Chan reports receiving grant support from Parvus Therapeutics on behalf of the University of Toronto, cofounding Cytodiagnostics and the Fio Corporation and holding equity in both companies, and having received fees for serving on the Scientific Advisory Board of Sigma-Aldrich Biotechnology in 2008–2009.
Supplementary Appendix

REFERENCES
. 1 Terminology for nanomaterials. Publicly available specification 136. London: British Standards Institute, 2007. (http://www.nanointeract.net/x/file/PAS%20136.pdf.)
. 2 Xia Y, Xiong YJ, Lim B, Skrabalak SE. Shape-controlled synthesis of metal nanocrystals: simple chemistry meets complex physics? Angew Chem Int Ed Engl 2009;48:60-103 CrossRef | Web of Science | Medline
. 3 Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol 2007;2:751-760 CrossRef | Web of Science
. 4 Council of the Canadian Academies. Small is different: a science perspective on the regulatory challenges of the nanoscale. July 2008. (http://www.nanolawreport.com/JulyCanadaReport.pdf.)
. 5 Thorek DLJ, Chen A, Czupryna J, Tsourkas A. Superparamagnetic iron oxide nanoparticle probes for molecular imaging. Ann Biomed Eng 2006;34:23-38 CrossRef | Web of Science | Medline
. 6 Resch-Genger U, Grabolle M, Cavaliere-Jaricot S, Nitschke R, Nann T. Quantum dots versus organic dyes as fluorescent labels. Nat Methods 2008;5:763-775 CrossRef | Web of Science | Medline
. 7 McCarthy TD, Karellas P, Henderson SA, et al. Dendrimers as drugs: discovery and preclinical and clinical development of dendrimer-based microbicides for HIV and STI prevention. Mol Pharm 2005;2:312-318 CrossRef | Medline
. 8 Davis ME, Zuckerman JE, Choi CHJ, et al. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature 2010;464:1067-1070 CrossRef | Web of Science | Medline
. 9 Sperling RA, Gil PR, Zhang F, Zanella M, Parak WJ. Biological applications of gold nanoparticles. Chem Soc Rev 2008;37:1896-1908 CrossRef | Web of Science | Medline
. 10 Liu ZA, Li XL, Tabakman SM, Jiang KL, Fan SS, Dai HJ. Multiplexed multicolor Raman imaging of live cells with isotopically modified single walled carbon nanotubes. J Am Chem Soc 2008;130:13540-13541 CrossRef | Web of Science | Medline
. 11 Hobbs SK, Monsky WL, Yuan F, et al. Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc Natl Acad Sci U S A 1998;95:4607-4612 CrossRef | Web of Science | Medline
. 12 Prencipe G, Tabakman SM, Welsher K, et al. PEG branched polymer for functionalization of nanomaterials with ultralong blood circulation. J Am Chem Soc 2009;131:4783-4787 CrossRef | Web of Science | Medline
. 13 Alexis F, Pridgen E, Molnar LK, Farokhzad OC. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol Pharm 2008;5:505-515 CrossRef | Medline
. 14 Libutti SK, Paciotti GF, Myer L, et al. Preliminary results of a phase I clinical trial of CYT-6091: a pegylated colloidal gold-TNF nanomedicine. J Clin Oncol 2007;25:Suppl:163s-163s
. 15 Visaria RK, Griffin RJ, Williams BW, et al. Enhancement of tumor thermal therapy using gold nanoparticle-assisted tumor necrosis factor-alpha delivery. Mol Cancer Ther 2006;5:1014-1020 CrossRef | Web of Science | Medline
. 16 Kim TY, Kim DW, Chung JY, et al. Phase I and pharmacokinetic study of Genexol-PM, a cremophor-free, polymeric micelle-formulated paclitaxel, in patients with advanced malignancies. Clin Cancer Res 2004;10:3708-3716 CrossRef | Web of Science | Medline
. 17 Harisinghani MG, Barentsz J, Hahn PF, et al. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. N Engl J Med 2003;348:2491-2499 Full Text | Web of Science | Medline
. 18 Enochs WS, Harsh G, Hochberg F, Weissleder R. Improved delineation of human brain tumors on MR images using a long-circulating, superparamagnetic iron oxide agent. J Magn Reson Imaging 1999;9:228-232 CrossRef | Medline
. 19 Perrault SD, Walkey C, Jennings T, Fischer HC, Chan WC. Mediating tumor targeting efficiency of nanoparticles through design. Nano Lett 2009;9:1909-1915 CrossRef | Web of Science | Medline
. 20 Posthuma-Trumpie GA, Korf J, van Amerongen AV. Lateral flow (immuno)assay: its strengths, weaknesses, opportunities and threats: a literature survey. Anal Bioanal Chem 2009;393:569-582 CrossRef | Web of Science | Medline
. 21 Nam JM, Thaxton CS, Mirkin CA. Nanoparticle-based bio-bar codes for the ultrasensitive detection of proteins. Science 2003;301:1884-1886 CrossRef | Web of Science | Medline
. 22 Lefferts JA, Jannetto P, Tsongalis GJ. Evaluation of the Nanosphere Verigene System and the Verigene F5/F2/MTHFR Nucleic Acid Tests. Exp Mol Pathol 2009;87:105-108 CrossRef | Web of Science | Medline
. 23 Thaxton CS, Elghanian R, Thomas AD, et al. Nanoparticle-based bio-barcode assay redefines “undetectable” PSA and biochemical recurrence after radical prostatectomy. Proc Natl Acad Sci U S A 2009;106:18437-18442 CrossRef | Web of Science | Medline
. 24 Hirsch LR, Stafford RJ, Bankson JA, et al. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc Natl Acad Sci U S A 2003;100:13549-13554 CrossRef | Web of Science | Medline
. 25 Huang X, Neretina S, El-Sayed MA. Gold nanorods: from synthesis and properties to biological and biomedical applications. Adv Mater 2009;21:4880-4910 CrossRef | Web of Science
. 26 Helms B, Meijer EW. Dendrimers at work. Science 2006;313:929-930 CrossRef | Web of Science | Medline
. 27 Nel AE, Madler L, Velegol D, et al. Understanding biophysicochemical interactions at the nano-bio interface. Nat Mater 2009;8:543-557 CrossRef | Web of Science | Medline
. 28 Derfus AM, Chan WCW, Bhatia SN. Probing the cytotoxicity of semiconductor quantum dots. Nano Lett 2004;4:11-18 CrossRef | Web of Science
. 29 Poland CA, Duffin R, Kinloch I, et al. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat Nanotechnol 2008;3:423-428 CrossRef | Web of Science
. 30 Hediel JD, Yu Z, Liu JYC, et al. Administration in non-human primates of escalating intravenous doses of targeted nanoparticles containing ribonucleotide reductase sub-unit M2 siRNA. Proc Natl Acad Sci U S A 2007;104:5715-5721 CrossRef | Medline
. 31 Johannsen M, Gneveckow U, Taymoorian K, et al. Morbidity and quality of life during thermotherapy using magnetic nanoparticles in locally recurrent prostate cancer: results of a prospective phase I trial. Int J Hyperthermia 2007;23:315-323 CrossRef | Web of Science | Medline
. 32 Schipper ML, Nakayama-Ratchford N, Davis CR, et al. A pilot toxicology study of single-walled carbon nanotubes in a small sample of mice. Nat Nanotechnol 2008;3:216-221 CrossRef | Web of Science
. 33 Hauck TS, Anderson RE, Newbigging S, Chan WCW. In vivo quantum dot toxicity assessment. Small 2010;6:138-144 CrossRef | Web of Science | Medline
. 34 Lidke DS, Nagy P, Heintzmann R, et al. Quantum dot ligands provide new insights into erbB/HER receptor-mediated signal transduction. Nat Biotechnol 2004;22:198-203 CrossRef | Web of Science | Medline
. 35 Chattopadhyay PK, Price DA, Harper TF, et al. Quantum dot semiconductor nanocrystals for immunophenotyping by polychromatic flow cytometry. Nat Med 2006;12:972-977 CrossRef | Web of Science | Medline
. 36 Kim S, Lim YT, Soltesz EG, et al. Near-infrared fluorescent type II quantum dots for sentinel lymph node mapping. Nat Biotechnol 2004;22:93-97 CrossRef | Web of Science | Medline
. 37 Gao X, Cui Y, Levenson R, Chung L, Nie S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat Biotechnol 2004;22:969-976 CrossRef | Web of Science | Medline
. 38 Suh WH, Suh Y-H, Stucky GD. Multifunctional nanosystems at the interface of physical and life sciences. Nano Today 2009;4:27-36 CrossRef | Web of Science
. 39 Park JH, Gu L, von Maltzahn G, Ruoslahti E, Bhatia SN, Sailor MJ. Biodegradable luminescent porous silicon nanoparticles for in vivo applications. Nat Mater 2009;8:331-336 CrossRef | Web of Science | Medline
40 Gharbi N, Pressac M, Hadchouel M, Szwarc H, Wilson SR, Moussa F. [60]fullerene is a powerful antioxidant in vivo with no acute or subacute toxicity. Nano Lett 2005;5:2578-2585 CrossRef | Web of Science | Medline





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 