Heart disease remains the most common cause of death and disability in our society. However, the face of this disease has evolved considerably in the decades since cardiovascular scientists began to understand the cellular and molecular mechanisms of its pathophysiology. Today, nearly 90% of patients hospitalized for a heart attack not only survive but also return to their normal activities and work within weeks, if not sooner — a vast improvement in outcome as compared with decades earlier.1-4 However, the evolution in the treatment of acute cardiovascular disease has also been paralleled by an increase in the number of patients with chronic debilitation due to heart failure.5 Despite advances in our understanding of the neurohormonal basis of heart failure, current therapies for heart failure are limited, and the need for additional therapies remains great.
Protein homeostasis plays a role in the development of numerous disorders. Misfolded proteins are central in the pathophysiology of neurodegenerative diseases such as Huntington's disease, Parkinson's disease, and Alzheimer's disease. In the past several years, misfolded proteins have been found to play a role in the pathophysiology of common human cardiac diseases such as pathologic cardiac hypertrophy and dilated and ischemic cardiomyopathies, leading to the suggestion that protein misfolding is a key contributor to the progression of heart failure. In this review, we explore the contribution of protein misfolding to the pathophysiology of cardiac disease, describing why these proteins become misfolded and how the innate systems that usually dispose of them break down. We then discuss how the knowledge obtained from studying protein misfolding in other diseases, such as Alzheimer's disease, may aid us in understanding the pathophysiological mechanisms of cardiac diseases and developing new treatments that focus on preventing or reversing protein misfolding in the heart.
PROTEIN QUALITY CONTROL IN THE HEART
The heart is a functional syncytium, propagating electrical impulses between communicating cells so that the complex and varied components of the heart can act as a single contractile apparatus. Cardiomyocytes, the contractile cells that make up the cardiac muscle, form a syncytium of unified contractile function. Thus, the maintenance of cardiomyocyte health is critical if the cardiac syncytium is to remain intact. Cardiomyocytes are made up of highly specialized components, such as sarcomeres, numerous mitochondria, and an extensive array of junctional complexes, that are responsible for maintaining adequate biochemical stores to meet the incessant energy demands placed on these cells during normal cardiac function. As terminally differentiated cells, cardiomyocytes have a very limited regenerative potential.6 Therefore, quality-control mechanisms for replacing proteins as they undergo wear and tear are necessary for the health and survival of cardiomyocytes. Optimal cardiomyocyte function depends on a critical balance among protein synthesis, protein folding, and protein turnover (Figure 1).
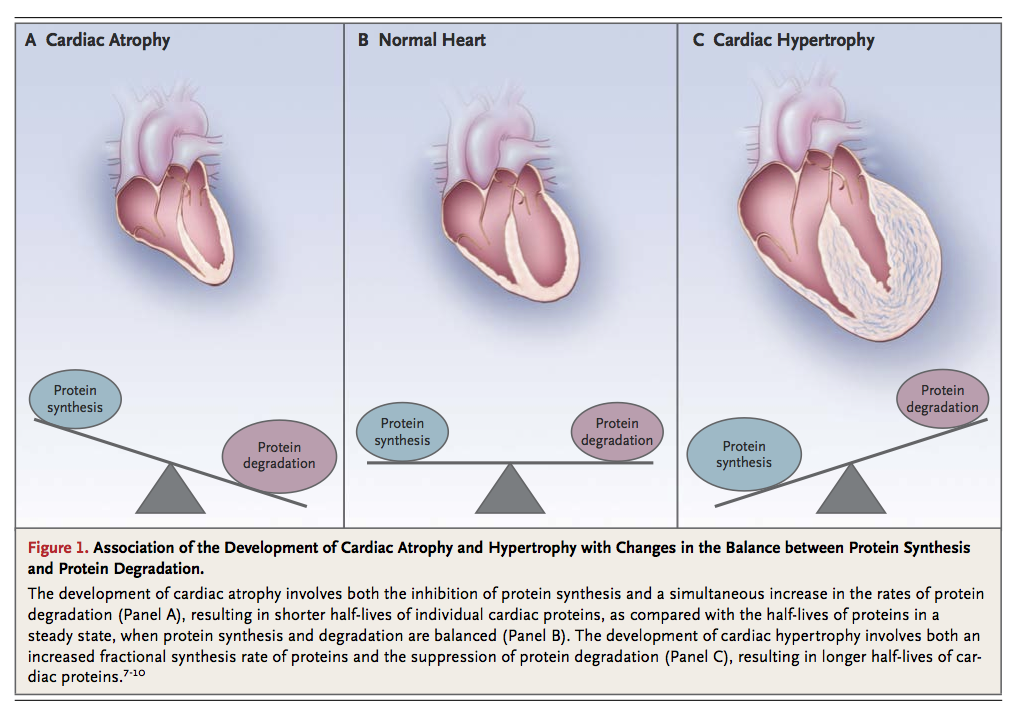
Molecular Chaperones as Regulators of Protein Synthesis and Folding
Newly synthesized translated proteins associate with molecular chaperones, which are specialized proteins that stabilize and fold nascent proteins. Several classes of chaperones and folding enzymes interact with unfolded nascent proteins to minimize aggregation.11 Molecular chaperones are highly abundant in cardiomyocytes. Some of these chaperones are specific to cardiac muscle, whereas others are present in other tissues as well.
Several members of the heat-shock protein (HSP) family of chaperones are expressed in the heart,12 including many small HSPs, such as HSPB5, also known as αB-crystallin (CryAB).13,14 The most abundant chaperone in the heart, CryAB associates with cytoskeletal and contractile-apparatus proteins in cardiomyocytes.15,16 In particular, it interacts with desmin, a critical protein in cardiomyocytes, to prevent its misfolding and subsequent aggregation.17 Mutations in CryAB, as discussed below, are linked to certain cardiomyopathies — specifically, desmin-related cardiomyopathies.18 The chaperone UNC-45 is critical for cardiomyocyte function through its role in regulating myosin folding and assembly and preventing its aggregation.19 Numerous other molecular chaperones associate with and protect the integrity of sarcomeric and infrastructural proteins in cardiomyocytes, including GimC (prefoldin) and TRiC (TCP-1 ring complex), both of which ensure proper folding and assembling of actin.20 Together, molecular chaperones are essential for protein quality-control mechanisms that ensure proper cardiac function by preventing cell death that can be caused by unfolded or aggregating proteins.
The Ubiquitin–Proteasome System and the Process of Autophagy
Balancing the synthesis of new proteins in the heart is the proteolysis of misfolded or damaged proteins. In the contractile cells of the heart, these proteins targeted for degradation include sarcomeric proteins that are essential for the function and regulation of cardiac mass (Figure 1). Two coordinated systems are responsible for the turnover of damaged and worn proteins in the heart: the ubiquitin–proteasome system and the process of autophagy. The ubiquitin–proteasome system is a highly specific, coordinated cascade of enzymes that targets specific proteins and labels them with multiple ubiquitin molecules, which in turn serve as recognition signals for their subsequent degradation by the proteasome, a large catalytic protease that is found throughout the cell.21-23 Ubiquitin ligases confer substrate specificity to the process of ubiquitinylation and are the rate-limiting enzymes in the ubiquitin–proteasome system. At least nine ubiquitin ligases have been identified in the heart, including muscle RING-finger proteins 1, 2, and 3 (MuRF-1, MuRF-2, and MuRF-3); atrogin-1 (also called muscle atrophy F-box); C-terminal of HSP70-interacting protein (CHIP); and murine double minute 2 (MDM2).24 These proteins can degrade sarcomeric proteins, including the myosin heavy chain (MuRF-1 and MuRF-3)25 and troponin I (MuRF-1),26 to maintain the integrity of the contractile apparatus.
To ensure optimal protein quality control in the heart, the ubiquitin–proteasome system and the molecular chaperone system must work in concert. When sarcomeric proteins become misfolded owing to routine stresses such as mechanical damage and oxidative stress (wear and tear), chaperones (e.g., HSP90 and UNC-45 in the case of the myosin heavy chain)20 attempt to refold the damaged proteins. However, if this fails, the molecular chaperones present their target proteins to the appropriate ubiquitin ligase, which then proceeds to ubiquitinylate the damaged protein, thereby targeting it for destruction by the ubiquitin–proteasome system. Newly synthesized proteins replace the degraded proteins, a process that once again requires the assistance of chaperones20 (Figure 2).
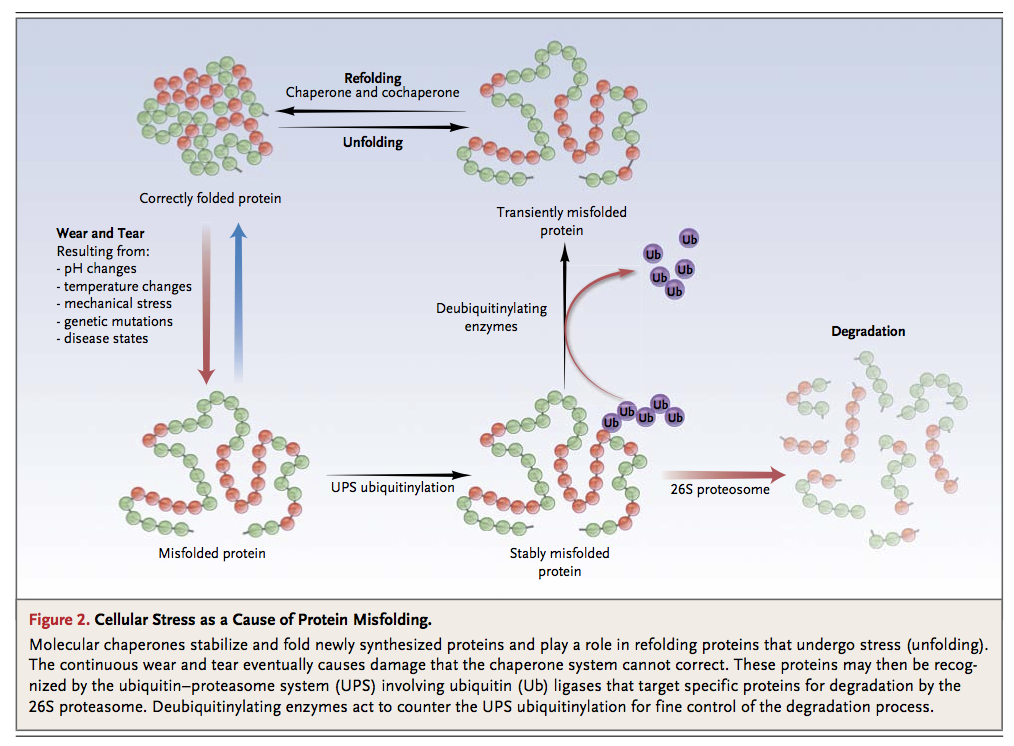
Autophagy is a second critical pathway for degrading proteins and maintaining protein quality control in the heart. Autophagy enhances the degradation of damaged organelles and aggregates of misfolded proteins by placing them in double-membrane–bound autophagic vacuoles that fuse with lysosomes, allowing their contents to be degraded by the acidic lysosomal hydrolases.27 In contrast to the specificity of the ubiquitin–proteasome system, autophagy is a relatively nonselective catabolic process that triggers protein degradation in the context of cellular stresses, such as nutrient deprivation.28 The importance of autophagy in maintaining physiologic homeostasis is illustrated by the fact that the failure of the autophagy system to remove protein aggregates has been linked to the development of certain neurodegenerative diseases, such as Parkinson's disease.29,30Autophagy is also associated with the pathophysiology of a number of cardiac disease states, including heart failure,5,31-34 ischemic heart disease,35-39 and chemotherapy-induced cardiomyopathy.40,41
Depending on their ubiquitinylation and aggregation status, misfolded proteins in the cytosol may be partitioned into one of two distinct cellular compartments.42 Misfolded proteins that are aggregated appear to be targeted to perivacuolar inclusions, where they are degraded through autophagy.42,43 In contrast, misfolded proteins that are ubiquitinylated, yet still soluble, tend to accumulate in juxtanuclear compartments enriched with proteasomes and to undergo proteolytic degradation. However, if the ubiquitin–proteasome system cannot meet the proteolytic demands of the cell because of an overload of misfolded proteins or impairment of the proteasome, accumulated ubiquitinylated proteins can be sequestered and degraded through autophagy; this demonstrates the cooperation between the two proteolytic systems.
PROTEIN AGGREGATION IN THE HEART
Proteotoxicity
A plethora of physiologic and pathologic stresses induce cellular proteins to misfold, including perturbations in pH, temperature, and osmotic pressure; mechanical strain; oxidative stress; and alterations in the genetic code. Misfolded proteins associate with one another to form sequentially higher orders of protein aggregates, such as soluble oligomers, soluble aggregates, and eventually, inclusion bodies (Figure 3
).
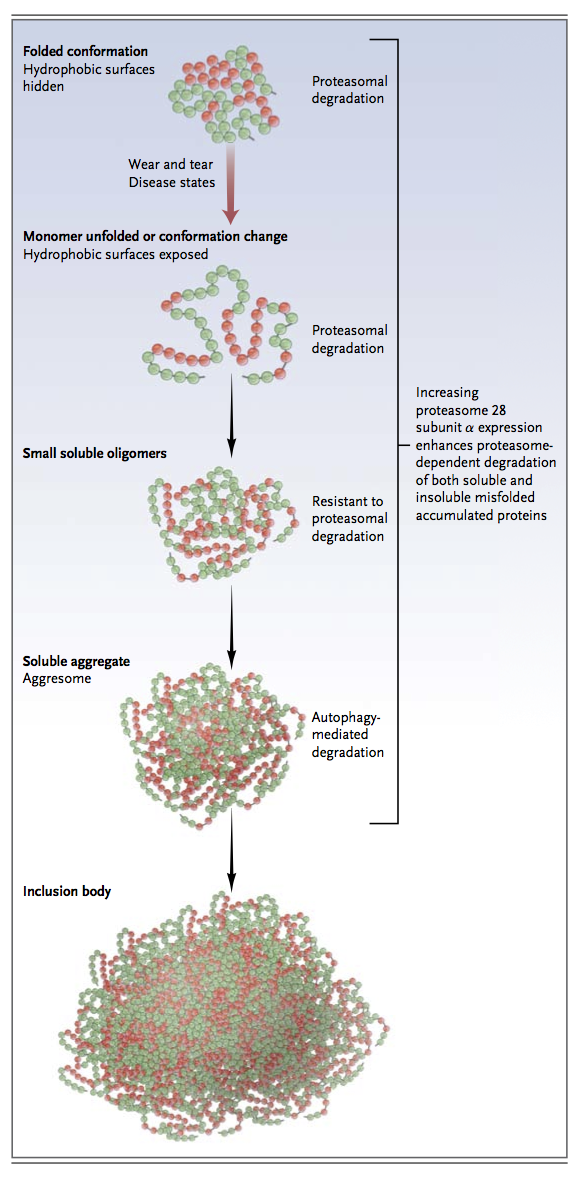
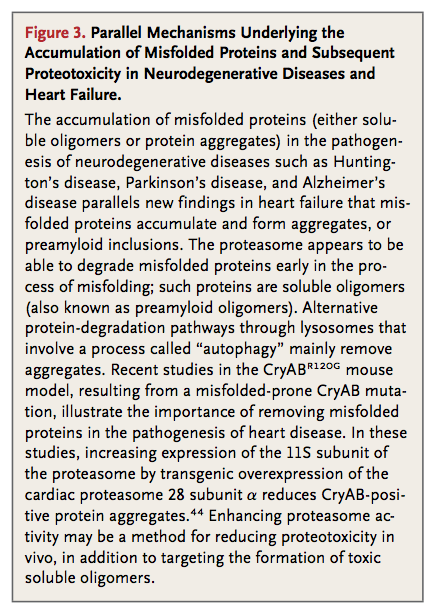
In neurodegenerative diseases, the presence of insoluble protein aggregates (fibrillar deposits) does not necessarily correlate with disease severity. This observation has led to the discovery that soluble protein oligomers can also be toxic.45,46Experimental evidence suggests that protein aggregates, or any one of the preceding intermediaries, may induce cell death, a process termed proteotoxicity. Proteotoxicity has been implicated in the pathogenesis of Alzheimer's disease and other proteinopathies such as Huntington's disease and Parkinson's disease.
Soluble oligomers and aggregated proteins disrupt cellular function in a number of ways. Soluble oligomers can interfere with proteasome function, disrupt normal cellular processes by binding critical cell-signaling and cell-trafficking molecules, and, in extreme cases, send out signals that result in cell death.47,48 Which specific intermediates are responsible for proteotoxicity remains a matter of controversy, because toxicity has been attributed to both soluble misfolded oligomers and aggregated proteins.49-51 However, fully aggregated proteins have been reported to be inert in some cases, and the process of sequestering aggregated proteins may be a defense mechanism of the cell.52,53
Proteotoxicity in Heart Failure
The accumulation of misfolded soluble protein oligomers is not restricted to neurodegenerative diseases. Cardiac stress disorders, such as dilated and hypertrophic cardiomyopathies, are associated with the presence of oligomeric protein depositions in cardiomyocytes. Soluble oligomers have been identified in cardiomyocytes from patients with hypertrophic cardiomyopathy, idiopathic dilated cardiomyopathy, and Becker's muscular dystrophy but not in those from healthy controls.54 Cytosolic aggregates are also found in cardiomyocytes from the hearts of patients with dilated cardiomyopathy. These aggregates are immunoreactive for ubiquitin and provide evidence of increased measures of autophagy.33
The presence of oligomer aggregates in response to cardiac stress has been further borne out in preclinical studies in mice. The induction of cardiac hypertrophy by pressure overload (transaortic constriction) in mice induces rapid formation of aggregates of misfolded proteins and aggresomes (inclusion bodies that form when the protein-degradation machinery is impaired or overwhelmed) in cardiomyocytes.55 Similarly, when isolated mouse cardiomyocytes are stressed through inhibition of proteasome activity, polyubiquitinylated proteins aggregate, a process that is associated with an increase in autophagic activity in the stressed cells. If autophagy is attenuated, the size and number of aggresomes increases, demonstrating that autophagy is a crucial mechanism by which cells control the level of accumulated protein aggregates.55 Although these studies show a clear correlative relationship between cardiac stress and the accumulation of protein aggregates, ubiquitinylated proteins, and the induction of autophagy, they do not definitively link the accumulation of misfolded proteins with heart failure.
Proteotoxicity in Hereditary Cardiac Disease
Desmin-related cardiomyopathy is a rare but well-defined genetic disorder caused by mutations in desmin or its chaperone, CryAB, resulting in the increased susceptibility to misfolding of both these proteins.54,56,57 The disorder is an illustrative example of how misfolded proteins can be causally associated with severe cardiac dysfunction and severe dilated cardiomyopathy.17,54,56,57Clinically, patients with desminopathies exhibit slowly progressive muscle weakness, cardiac arrhythmias, conduction-system disease, and cardiomyopathy.58
The most common form of desmin-related cardiomyopathy results from a missense mutation in CryAB (CryABR120G).18 Many of the features of human disease are recapitulated in mice in which this same CryABR120G mutation is expressed in the heart. Cardiac CryABR120G mutation expression in transgenic mice results in a dilated cardiomyopathy and the formation of electron-dense aggresomes characteristic of those formed in neurodegenerative diseases.54 These developing aggregates contain CryAB, desmin, and ubiquitin, and increases in autophagic activity are seen.54,59-61 Dysregulation in the ubiquitin–proteasome system occurs in CryABR120Gtransgenic mice before any impairment in cardiac function is noted, perhaps because the accumulated misfolded proteins poison the proteasome.54 Although it is not yet known which of the protein-aggregate intermediates (Figure 3) may be responsible for decrements in cardiac function in patients with desmin-related cardiomyopathy, these observations show that defects in protein folding alone can lead to heart failure in mouse models.
Preclinical studies have helped to establish a broader relationship between defects in protein quality control and cardiac dysfunction. For example, in mice lacking the MuRF family of ubiquitin ligases, which maintain quality control of the cardiac sarcomere, an exaggerated cardiac hypertrophy develops (MuRF-1−/−), whereas increased cardiac expression of MuRF-1 leads to an increased susceptibility to heart failure in the context of pressure-overload–induced cardiac hypertrophy.62,63 The toxic effects of misfolded proteins on cardiac function, akin to the neurologic toxic effects of preamyloid oligomers, have been tested in mouse hearts that express small oligomers prone to misfolding (called PQ83) or a non–oligomer-forming peptide (called PQ19).64In mice with cardiac expression of the small soluble oligomer PQ83, heart failure developed by 5 months of age, whereas in mice with cardiac expression of the non-aggregating control protein PQ19, cardiac function was unaffected.64
By linking either defects in pathways that protect against protein misfolding or the accumulation of misfolded proteins directly with the development of cardiomyopathy, these studies offer clinicians a new way to think about how protein aggregation — which occurs in heart failure, ischemic heart disease,65,66 and specific genetic diseases associated with cardiac dysfunction — plays an independent and pivotal role in the pathogenesis of heart failure. New strategies to target protein misfolding are being developed to treat common cardiovascular diseases.
THERAPEUTIC IMPLICATIONS
Our increased understanding of the role that protein aggregation plays in proteotoxicity and, ultimately, the development of left ventricular dysfunction and heart failure provides the basis for designing therapeutic strategies to improve cardiac performance. Specifically, therapies that reduce the accumulation of misfolded proteins, their toxic effects, or both may be helpful in patients with congestive heart failure.
Increasing the Expression of Small HSPS in Cardiac Disease
The small HSP CryAB functions as part of larger molecular complexes that contain proteins requiring assistance with folding as well as other HSPs, such as HSPB1 and HSPB8.67,68 When the level of HSPB1 is increased experimentally in cardiac cells in culture, the solubility of misfolded CryAB aggregates is enhanced and the number of aggregates decreases.59 As a consequence, HSPB1 facilitates the ubiquitinylation and proteasomal degradation of protein aggregates caused by multiple CryAB misfolding mutations,59 showing that increasing HSP expression can enhance both the solubility and degradation of misfolded proteins in cardiomyocytes.
Similarly, when HSPB8 and HSPB1 expression is increased by means of the drug geranylgeranylacetone in the CryABR120G transgenic mouse model of desmin-related cardiomyopathy, the progression of heart failure is significantly inhibited.69 Treatment with geranylgeranylacetone also reduces the formation of amyloid oligomers and insoluble aggregates,69 leading to a reduction in heart size, a decrease in fibrosis, recovery of cardiac function, and improved overall survival; these observations suggest that the induction of small HSPs may be beneficial in cardiomyopathies caused by mutations in CryAB. Geranylgeranylacetone, which has been approved for the treatment of ulcers, might also be used to reduce the burden of misfolded proteins in the heart that contribute to heart failure.69,70
When HSPA4 (which is necessary for the maintenance of HSP70) is disrupted, misfolded proteins accumulate in the heart, and spontaneous cardiac hypertrophy develops.71 Thus, HSPs are necessary for the maintenance of cardiac integrity, and increasing the expression of small HSPs is a potential therapeutic strategy for modifying the protein aggregates caused by mutations in CryAB or in more common forms of heart failure in which misfolded proteins accumulate. Taken together, these studies suggest that the manipulation of the expression of small HSPs in the heart with drugs such as geranylgeranylacetone may hold promise for halting the progression of heart failure due to misfolded proteins, leading to improved cardiac function and survival.
Exercise
Environmental enrichment with voluntary exercise have proved beneficial in the treatment of neurodegenerative diseases associated with misfolded proteins.72-75 For example, voluntary running in the Huntington's disease R6/1 mouse model (in which exon 1 of the human Huntington's disease gene is expressed with an expanded CAG repeat, creating a polyglutamine tract) decreases neural amyloid deposits and enhances learning ability, as compared with findings in littermates that do not run.76,77 This improved cognition has been linked to changes in the activity of cerebrolysin, an enzyme that reduces amyloid deposition either by enhancing the degradation of amyloid-beta or by inhibiting the expression, maturation, or processing of amyloid precursor protein.78
Several parallels between CryABR120G desmin-related cardiomyopathy and neurodegenerative diseases have been identified. The cytoplasmic accumulation of misfolded proteins found in inclusion bodies has been identified in the CryABR120G transgenic-mouse model.54 Antibodies that recognize the toxic conformation shared with preamyloid oligomers and amyloidogenic proteins79 also recognize material in CryABR120G cardiomyocytes.54,80 Furthermore, the severity of disease in CryABR120G cardiomyopathy correlates with the amount of antibody-reactive material,54,80 not with the amount of aggresomes or amyloid-positive plaques, a finding that suggests a pathogenetic role of preamyloid oligomers.81 On the basis of similarities in the preamyloid oligomers found in cardiomyopathy associated with the CryABR120G mutation and in neurodegenerative diseases such as Alzheimer's disease, it has been hypothesized that exercise may also improve cardiac function and survival in patients with CryABR120G-associated cardiomyopathy.81 Indeed, when CryABR120G transgenic mice are encouraged to exercise voluntarily, reductions in the deposition of cardiac amyloid oligomers and in signs of heart failure are observed, and the premature death normally associated with this mutation is averted.81
The mechanism behind this exercise-induced rescue appears to be due, in part, to the maintenance of the beneficial effect of neprilysin in the heart.81 Neprilysin, like cerebrolysin, decreases the accumulation of preamyloid oligomers.81 Whereas the CryABR120G mutation decreases the level of neprilysin, possibly accounting for the increase in preamyloid oligomers in these mice, exercise maintains wild-type levels, suggesting that exercise may provide protection against the progression of cardiac disease to heart failure by increasing neprilysin and decreasing cardiac amyloid accumulation in this transgenic-mouse model.
These studies highlight the benefits of exercise in maintaining protein folding in the heart and preventing premature death. These findings parallel those from the growing number of studies in animals and humans that show the beneficial effects of exercise on cognitive function in Alzheimer's disease,82 and they provide another way to think about the well-established benefits of exercise on cardiac function.
Novel Therapies for Diseases Associated with Misfolded Proteins
Although we have focused thus far on studies of neurodegenerative disease and their application to heart failure, the underlying pathophysiological processes that result from misfolded proteins have also been described in other common diseases. For example, the most common mutation that causes cystic fibrosis results in a functional, yet misfolded, protein. Specifically, mutations in the cystic fibrosis transmembrane conductance regulator gene (CFTR, which encodes an epithelial chloride channel that is essential for the proper functioning of the pancreas, lung, and other organs) result in a misfolded protein, which is recognized by the ubiquitin–proteasome system in the endoplasmic reticulum and targeted for degradation. This defective processing of the CFTR protein and subsequent loss of CFTR expression on the cell surface are responsible for the clinical manifestations of the disease.83-85
Small-molecule compounds that can correct the misfolding of mutant CFTR have been developed for therapeutic purposes to normalize CFTR expression on the cell surface. One such molecule, VX-809, is showing great promise in clinical trials involving patients with cystic fibrosis.86 VX-809 promotes the processing and cell-surface expression of CFTR mutants (which are functional, despite their mutations) in human bronchial epithelial cells. VX-809 and a related compound, VX-770, are now being tested in exploratory phase 2 clinical trials, in which they appear to increase the cell-surface expression of the mutant (ClinicalTrials.gov number, NCT01225211).87 Although the use of VX-809 has been limited to cystic fibrosis, drugs such as these that promote protein folding may have application in heart failure and other cardiac diseases associated with protein accumulation that is caused by defective protein processing.
High-throughput screening has been used to search for drugs that can reduce proteotoxicity and that may be applicable to the treatment of heart failure.88 In the context of neuronal proteotoxicity, drug screening has identified numerous compounds that attenuate proteotoxic effects of high glucose levels and aging in neurons,89 whereas other high-throughput screening experiments have identified chemicals in the quinoxaline90 and anthraquinone91 families that decrease the formation of tau-protein aggregates in vitro and in cell culture. As these and other novel compounds are discovered and characterized for their application in combating neuronal proteotoxicity, their usefulness in heart failure should also be considered, given the emerging evidence of the role that protein aggregation and proteotoxicity play in many common cardiovascular diseases.
CONCLUSIONS AND FUTURE DIRECTIONS
Despite large increases in the number of patients with chronic congestive heart failure, there have been few innovations in pharmacologic approaches to treat it. A new understanding of the role of proteotoxicity in the heart and its direct effects on cardiac performance suggests that targeting the accumulation of misfolded proteins, ameliorating their effects, or both may be beneficial in patients with heart failure. Multiple studies have shown potential ways to prevent this accumulation, including enhancement of the expression of small HSPs and exercise. However, it is critical to raise the awareness that wear and tear in the heart due to protein misfolding is a major and targetable contributor to cardiac dysfunction.





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 