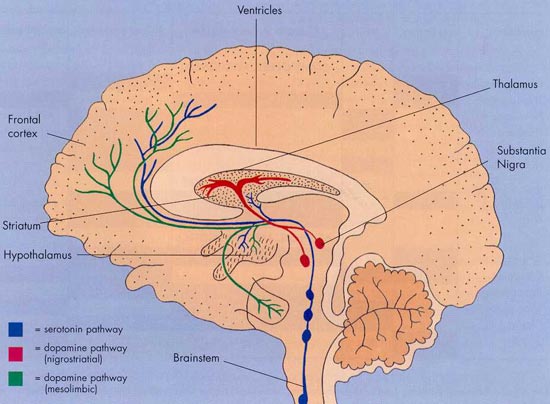
機轉圖也可以參考這邊:
圖解藥理學~22Dopamine pathway
Rasagiline is a second-generation irreversible monoamine oxidase type B (MAO-B) inhibitor indicated for the treatment of Parkinson's disease (PD) and the product of a joint development programme between the Danish pharmaceutical company Lundbeck and Teva Pharmaceutical of Israel.
Following successful completion of phase III trials in patients with early and advanced PD, rasagiline was filed for regulatory approval in both the US and Europe in 2003.
In February 2005 the European Medicines Agency (EMEA) gave final marketing authorisation for its use as initial monotherapy in patients with early PD and as adjunctive treatment in those with moderate-to-advanced PD. FDA approval followed in 2006.
Lundbeck and Teva jointly market rasagiline under the brand name Azilect in Europe as part of the two companies' long-term strategic alliance. Teva Neuroscience markets Azilect in the US, following the ending of a co-development and marketing agreement with Eisai.
Improved treatments are needed for PD
PD is named after Dr James Parkinson, a physician who first described the symptoms of "shaking palsy" in 1817. Estimated to affect over 4 million people worldwide, it is the second most common neurodegenerative disease after Alzheimer's disease. It is a progressive neurological disorder characterised by tremor, stiffness in the limbs, slow movement as well as impaired balance and co-ordination. Primarily a disease of the elderly, its prevalence is projected to rise as the proportion of elderly people in the Western population continues to increase.
PD is a disease of the basal ganglia arising from the loss of dopamine-producing cells in the substantia nigra. The loss of dopamine, a major neurotransmitter, produces the characteristic motor symptoms of PD. Current treatments aim to address the deficiency in dopamine production.
Levodopa, a naturally occurring amino acid, has been the mainstay of treatment for PD for the last 30 years. Although it remains the gold standard, over time as many as three-quarters of patients on levodopa develop long-term side effects such as dyskinesias (involuntary movements), "on-off" motor syndromes and mental disturbances such as hallucinations.
While the ultimate goal of treatment is to cure the disease, new drugs that can offer improved symptomatic relief are greatly needed. MAO-B inhibitors are an important new class of drugs for symptomatic relief of PD. As their name implies they inhibit MAO-B, an enzyme responsible for the breakdown of dopamine. By preventing dopamine breakdown, MAO-B inhibitors may help reduce dopamine loss and delay the need for treatment with levodopa, thus increasing its clinical utility.
Currently only one MAO-B inhibitor, selegiline, is approved for treatment of PD. Approval of rasagiline, a second-generation compound, has therefore increased treatment options with this class of agents. Compared with selegiline, rasagiline exhibits more potent in vivo inhibition of MAO-B, and because its metabolism is different it appears devoid of the undesirable amphetamine-related effects seen with selegiline. These include increases in blood pressure, sleep disturbances and euphoria.
Clinical trials suggest rasagiline may delay progression of PD
The clinical efficacy and safety of rasagiline was evaluated in a series of phase III trials, in which it was administered alone or in combination with standard levodopa therapy. In the 26-week TEMPO trial, in which rasagiline monotherapy was compared with placebo in 404 patients with early PD (not on levodopa), treatment with rasagiline was significantly more effective than placebo with respect to change in the Unified Parkinson's Disease Rating Scale (UPDRS) score from baseline to endpoint.
When treatment was continued for a full 12 months, patients in the two active treatment arms (1mg and 2mg rasagiline) of the TEMPO trial showed a smaller decline in total UPDRS compared with those whose switch to active treatment was delayed by 6 months (placebo group). These findings suggest that early initiation of rasagiline may slow the progression of impairment associated with PD.
Efficacy was also demonstrated when rasagiline was given in conjunction with levodopa. In the 26-week placebo-controlled PRESTO trial involving 472 PD patients, rasagiline 1mg/day or 0.5mg/day given in conjunction with levodopa significantly reduced off-time compared with placebo as well as improving motor function. 'Off-time' is used to describe periods of poor overall function when the effects of levodopa wear off and symptoms return.
This is supported by data from the 697-patient LARGO trial, in which PD patients were randomised to 18 weeks of treatment with rasagiline, entacapone (a COMT inhibitor), and placebo in addition to levodopa.
Consistent with the PRESTO trial, treatment with rasagiline reduced off-time, with the magnitude of response similar to that seen with entacapone. In this study rasagiline was noted for its long duration of action.
More recently, preliminary data from the ADAGIO study also suggests that treatment with rasagiline may exert a neuroprotective effect and slow progression of PD. In this specially designed trial, patients were randomised into one of two groups: early active treatment and delayed start following placebo. After the initial treatment phase all patients received rasagiline.
At the end of the second treatment phase, benefit on measures of disease progression was observed in patients on early treatment but not those in the delayed treatment group. Investigators believe that these findings cannot be easily explained by a symptomatic effect, as both groups were on the same treatment, but are suggestive of neuroprotective effects.
Overall the clinical trial results suggest rasagiline is a well-tolerated drug with an incidence of adverse events similar to placebo. In one trial postural hypotension occurred with significantly greater frequency than placebo.
Marketing commentary
Although there have been some improvements in the treatment of PD over the past decade, there remain many unmet needs for patients with PD. Significant market opportunities therefore exist for drugs that can offer greater symptomatic improvement over existing agents as well as conferring some degree of neuroprotection.
Analysts are optimistic that the drug, which is administered orally and does not require titration, will be an important addition to the range of drugs available to treat patients with PD.





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 