實際標示就像是上圖這個樣子
The FDA published the Content and Format of Labeling for Human Prescription Drug and Biological Products; Requirements for Pregnancy and Lactation Labeling, referred to as the “Pregnancy and Lactation Labeling Rule” (PLLR or final rule).
The PLLR requires changes to the content and format for information presented in prescription drug labeling in the Physician Labeling Rule (PLR) format to assist health care providers in assessing benefit versus risk and in subsequent counseling of pregnant women and nursing mothers who need to take medication, thus allowing them to make informed and educated decisions for themselves and their children. The PLLR removes pregnancy letter categories – A, B, C, D and X. The PLLR also requires the label to be updated when information becomes outdated.
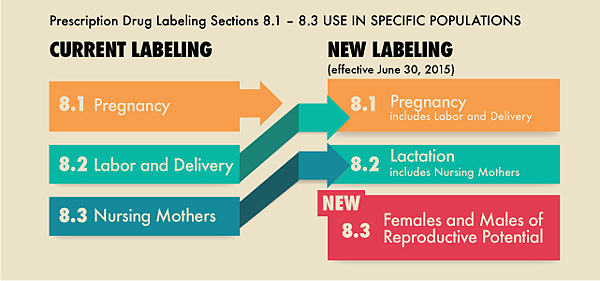
Below is a comparison of the current prescription drug labeling with the new PLLR labeling requirements.
FDA新版懷孕及授乳標示內容包含三大主題,分別為:
懷孕(Pregnancy):包含分娩與生產(Labour and Delivery )
授乳(Lactation):包含親餵母乳(Nursing Mothers)
對生殖力的影響(Females and Males of Reproduction Potential)
The Pregnancy subsection (8.1) includes information for a pregnancy exposure registry for the drug when one is available. Pregnancy exposure registries collect and maintain data on the effects of approved drugs that are prescribed to and used by pregnant women. Information about the existence of any pregnancy registries in drug labeling has been recommended but not required until now. Information in the Pregnancy sub-section includes a Risk Summary, Clinical considerations, and Data. Information formerly found in the “Labor and delivery” subsection is now included in the “Pregnancy” subsection.
The Nursing mothers subsection was renamed, the Lactation subsection (8.2), and provides information about using the drug while breastfeeding, such as the amount of drug in breast milk and potential effects on the breastfed infant.
The Females and Males of Reproductive Potential subsection (8.3), new to the labeling, includes information, when necessary, about the need for pregnancy testing, contraception recommendations, and information about infertility as it relates to the drug.
The labeling changes go into effect on June 30, 2015. Prescription drugs and biologic products submitted after June 30, 2015, will use the new format immediately, while labeling for prescription drugs approved on or after June 30, 2001, will be phased in gradually.
Labeling for over-the-counter (OTC) medicines will not change; OTC drug products are not affected by the final rule.
Concurrently with publishing the PLLR, FDA also issued draft guidance for industry to assist drug manufacturers in complying with the new labeling content and format requirements.
指引你可以自行下載
簡單中文整理如下:
1. 妊娠期(Pregnancy subsection, 8.1)
包括懷孕(妊娠)、產程與分娩(Labor and delivery),其中的資訊包括風險概要(Risk Summary)、臨床考量(Clinical considerations),以及資料(Data)。
內容包括妊娠期間藥物使用資訊,以及提供妊娠女性使用該藥物時收集和更新數據的相關登記訊息,亦即「懷孕暴露註冊試驗」(Pregnancy exposure registry)等四類子項目:
(1) 懷孕暴露註冊試驗(Pregnancy Exposure Registry)
「懷孕暴露註冊試驗」用於研究孕婦與新生兒服用藥物或施打疫苗時的健康資訊,並與未服用藥物的孕婦進行比對。
(2) 風險概要(Risk Summary)
a. 基於人類資料的風險聲明(Risk statement based on human data)
b. 基於動物資料的風險聲明(Risk statement based on animal data)
c. 基於藥理學的風險聲明(Risk statement based on pharmacology)
(3) 臨床考量(Clinical Considerations)
a. 與疾病相關的母體與/或胚胎(胎兒)風險(Disease-associated maternal and/or embryo/fetal risk)
b. 在妊娠期與產後的劑量調整(Dose adjustments during pregnancy and the postpartum period)
c. 對母體的不良反應(Maternal adverse reactions)
d. 對胎兒/新生兒的不良反應(Fetal/Neonatal adverse reactions)
e. 產程或分娩(Labor or delivery)
(4) 資料(Data)
a. 人類的資料
b. 動物的資料
2. 哺乳期(Lactation subsection, 8.2)
包括母乳餵養期間的藥品使用資訊,例如藥物在母乳中的含量及其對幼兒的潛在影響。
(1) 風險概要(Risk Summary)
a. 存在人體乳汁的藥物(Presence of drug in human milk)
b. 藥物對接受哺乳孩童的影響(Effects of drug on the breastfed child)
c. 藥物對乳汁產生與分泌的影響(Effects of drug on milk production/excretion)
d. 風險與利益聲明(Risk and benefit statement)
(2) 臨床考量(Clinical Considerations)
a. 儘量減少暴露(Minimizing exposure)
b. 監視不良反應(Monitoring for adverse reactions)
(3) 資料(Data)
3. 對女性和男性生殖系統影響(Females and Males of Reproductive Potential subsection, 8.3)
描述藥品對懷孕檢驗、避孕建議與不孕症方面的資訊。
(1) 懷孕檢驗(Pregnancy Testing)
(2) 避孕(Contraception)
(3) 不孕症(Infertility)







 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 