Emily Sexson, PharmD, BCPS, CDE
Assistant Professor of Pharmacy Practice
Creighton University School of Pharmacy and Health Professions
Omaha, Nebraska
Jon Knezevich, PharmD
Assistant Professor of Pharmacy Practice
Creighton University School of Pharmacy and Health Professions
Omaha, Nebraska
6/17/2010
US Pharm. 2010;35(6):HS7-HS16.


Male hypogonadism is defined as a testicular dysfunction resulting in decreased sperm and testosterone production.1 This disorder is not uncommon and frequently goes undiagnosed due to the nonspecific clinical presentation of the disease. However, male hypogonadism has been described in up to 20% of men over the age of 70 years and has been reported in 4% of all men.2,3 Trends demonstrating higher frequency of males affected by this disorder may be related to increased life expectancy, as rates of hypogonadism increase with age.2 In addition, the growing numbers of Americans who are overweight and who develop type 2 diabetes mellitus have also been described as potential causes for the increasing prevalence. Recent data have suggested that up to one-third of men with a diagnosis of type 2 diabetes and a body mass index (BMI) of >30 kg/m2 have hypogonadism defined by a low serum-free testosterone level. The cause for reduced androgen levels in these patients is unclear; however, there have been some proposed mechanisms for this observation.
Epidemiology
First, it is thought that androgens are extensively cleared in adipose tissue in obese patients. Furthermore, aromatization of androgens rises with increased levels of adipose tissue. Third, it is believed that in obese patients there is an increase in inflammatory mediators, which may decrease hormones that stimulate production of androgens (i.e., luteinizing hormone [LH] and gonadotropin-releasing hormone [GnRH]). This theory is questioned, however, when low androgen levels are observed in lean men with type 2 diabetes, demonstrating that hypogonadism in patients with diabetes may be independent of adiposity.3
Pathophysiology
Two categories of male hypogonadism have been described—primary and secondary. Hypergonadotropic hypogonadism or primary hypogonadism results from disease of the testes, which can be congenital or acquired. In primary hypogonadism, sperm production is more significantly affected than testosterone production due to extensive damage to the seminiferous tubules.
The most common form of congenital primary hypogonadism is Klinefelter’s syndrome. This genetic disorder occurs when a male is born with an additional X chromosome, and it is seen in an estimated 1 in 1,000 male births.2 Abnormalities that can occur with Klinefelter’s syndrome include damage to the seminiferous tubules and Leydig cells, resulting in decreased size of the testes as well as low sperm counts and testosterone levels. In addition to infertility caused by testosterone deficiency, men born with this disorder frequently have increased length of their long bones, including those in the arms, legs, and hands. Many other complications exist independent of the testosterone deficiency seen with these patients, including predisposition to developing respiratory conditions, carcinomas, and many other comorbidities, which are not preventable with testosterone replacement.
Acquired primary hypogonadism occurs when a patient is exposed to a substance, experiences trauma, or contracts a disease or infection that results in decreased sperm and testosterone production, often leading to infertility. Common medication causes resulting in this disorder include glucocorticoids, ketoconazole, and alkylating/neoplastic agents (e.g., cyclophosphamide, chlorambucil, cisplatin, and busulfan).4 Causes of both congenital and acquired primary hypogonadism are listed in TABLE 1.
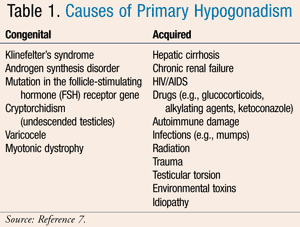
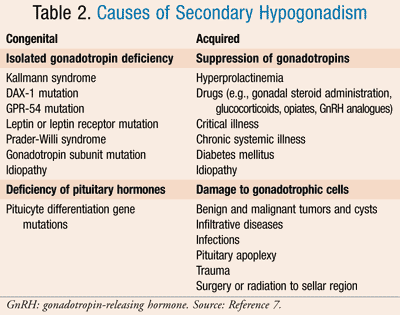
Secondary hypogonadism is labeled hypogonadotropic hypogonadism. This occurs with dysfunction of pituitary gonadotropin release or hypothalamic release of GnRH. As a result, testosterone and sperm counts are proportionately low while serum LH and follicle-stimulating hormone (FSH) are normal, identifying a problem with the stimulating pathway in androgen synthesis. Additionally, this disorder is characterized as either congenital or acquired. Congenital disease is rare and often associated with gene mutations inhibiting GnRH recognition. Acquired secondary hypogonadism is caused by any disease or substance that can alter the hypothalamic-pituitary-adrenal (HPA) axis. Medications that can increase the risk of developing this disorder include gonadal steroids, GnRH analogues, glucocorticoids, and chronic opiates.5 Additional causes of the secondary forms of hypogonadism are listed in TABLE 2.
Clinical Presentation
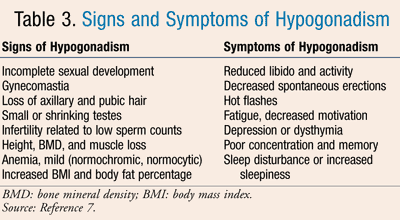
Clinical symptoms of hypogonadism vary depending on the severity and type of disease. In adults, symptoms can range from decreased libido and ability to perform sexually to height, bone mineral density, and muscle loss. Infertility, loss of body hair, and incomplete sexual development have been reported as well. Less-specific but still commonly described symptoms may include depressed mood, difficulty sleeping, mild anemia, fatigue, or even increased body fat and BMI.2,6,7 Objective examination must compare sexual development consistent with the patient’s age. A more thorough list of reported signs of hypogonadism is given in TABLE 3.
Diagnosis
Because symptoms are nonspecific in nature, a complete history, physical examination, and laboratory analysis must accompany the clinical presentation for diagnosis. A history that focuses on developmental milestones will help the clinician determine the onset of the disorder. Possible causes must also be ruled out during a patient history, such as exposure to environmental toxins, medications that have been associated with the disorder, or evidence of a congenital cause such as Klinefelter’s syndrome or pituitary gland hormone deficiency.4 In addition, patients should be asked about recreational drug use, eating disorders, and excessive exercise, as all of these can transiently alter testosterone levels.7
If clinical symptoms are present, serum testosterone levels should be measured. The total testosterone level should be the first measurement taken to determine hypogonadic state. It should be drawn between the hours of 8 am and 11 am to ensure that a peak level is captured (diurnal fluctuations can occur throughout the day, especially in younger men).7 If an abnormal laboratory value is identified, a confirmatory measurement should be performed to ensure that the diagnosis is appropriate. It has been reported that up to 30% of patients with a low testosterone level upon initial laboratory measurement have a normal level upon repeat measurement.7 Numerous factors can transiently alter testosterone levels, including time of measurement (day and year), medications, and acute illness.
In most references, a total testosterone level <300 ng/mL is considered low; however, some controversy surrounds the inconsistency with measurement assays in determining this reference range.7 Free testosterone should be measured if it is suspected that the patient may have alterations in his sex hormone–binding globulin (SHBG). Testosterone is highly bound to SHBG and albumin; thus, when SHBG concentrations are significantly affected, circulating testosterone levels can be as well. Many things can cause alterations in SHBG concentrations, some of which include obesity, nephrotic syndrome, hypothyroidism, and use of glucocorticoids, progestins, and androgenic steroids. Conditions associated with increased levels of SHBG include aging, hepatic cirrhosis, hyperthyroidism, HIV, and use of anticonvulsants or estrogens. The lower limit of normal when discussing free testosterone is typically 50 pg/mL.7
Other laboratory measurements that should be completed if testosterone levels return low include measurement of LH and FSH. These stimulatory hormones help clinicians determine whether the identified hypogonadism is of primary or secondary nature. As described previously, primary or hypergonadotropic hypogonadism would demonstrate an elevation in these hormones, signifying adequate pituitary function. On the other hand, if LH and FSH levels are low, a suspected pituitary cause must be considered, with further imaging necessary to rule out structural abnormalities.7
Treatment
Treatment strategies for both primary and secondary forms of male hypogonadism are similar and involve supplementation of testosterone or induction of endogenous testosterone production.2 Of the candidates appropriate to receive therapy, only 5% actually are prescribed testosterone replacement therapy (TRT).8 TRT is recommended for men with clinical symptoms of androgen deficiency combined with laboratory evidence of abnormally low levels of serum testosterone.7 However, testosterone therapy in patients with diagnoses of breast or prostate cancer is not recommended. This is also true in patients with an elevated prostate-specific antigen (PSA) >3 ng/mL, without further urologic workup.7
Goals of Therapy
Goals of treating hypogonadal patients are focused around improvement of those symptoms, many of which can be achieved with exogenous testosterone replacement. For patients with primary hypogonadism with decreased sperm counts, virility is commonly not achieved despite supplementation with testosterone products. Thus, GnRH production must be stimulated through use of pulsatile GnRH or treatment with exogenous gonadotropins. Choice of agent is dependent on the cause of the disorder. If the problem stems from the hypothalamus, GnRH administration is appropriate, whereas exogenous gonadotropins should be utilized for patients with a combination of pituitary and hypothalamic disorders.2
In addition to improvement of clinical symptoms, laboratory goals are also set to ensure efficacy. Clinicians should monitor total testosterone levels and aim for values in the normal range for adults based upon the patient’s age and the assay used for measurement. Free and bioavailable testosterone levels should be determined if an altered SHBG is suspected.7
Available Products
Androgen therapy in the form of TRT is the most commonly used treatment modality for patients with hypogonadism. There are a variety of delivery forms available for providing testosterone replacement. A review of testosterone supplements is presented in TABLE 4.
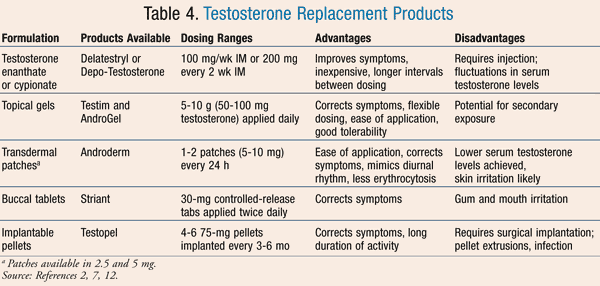
Topical Gels: Two topical gels are currently available in the U.S. that are approved for the treatment of male hypogonadism. AndroGel and Testim are both 1% gels dispensed as 5- to 10-g tubes or in packets containing 50 to 100 mg of testosterone. Daily administration is believed to allow the patient 10% absorption into the systemic circulation.8 Patients should be advised to apply the appropriate quantity of gel to clean, dry, intact skin of the upper arms, shoulders, or abdomen. Caution should be taken after administration to avoid skin contact with others. Although testosterone transmission with skin contact is not significant, secondary exposure has been reported, and there is a black box warning regarding the risks if this transfer occurs in children, which may cause penile or clitoral enlargement, premature development, and aggressive behavior.1 To prevent this potential hazard, patients should be advised to wash hands thoroughly after administration, allow the administration area to dry completely before contact occurs, and wear clothing over the area where the gel was delivered. Skin irritation has been described, although it is not common. Testosterone gels have anecdotally been reported to emit a musky odor that may be bothersome to some patients.
The positive effects on symptom control and the general tolerability and ease of their use make testosterone gels often the agent of choice when selecting a testosterone product. Clinical studies have demonstrated that, when compared to transdermal patches, gels allow for longer-lasting serum testosterone levels in a eugonadal range. This has been associated with improvements in sexual function, body composition, mood, and bone density.8 Testosterone gels do not undergo first-pass metabolism and thus have not been associated with hepatic toxicity.2
Transdermal Patches: The transdermal product Androderm is currently the only FDA-approved transdermal patch delivery system for testosterone replacement. Patches are available in 2.5- and 5-mg strengths, and testosterone is delivered over a 24-hour period, requiring patients to change the patch daily.8 Improved absorption has been noted when patients apply patches to the back, thigh, upper arm, or abdomen, where it has been reported that 4 to 5 mg is absorbed after placement of two 2.5-mg patches daily. Lower absorption occurs if patches are placed elsewhere.8 Scrotal patches are available in other parts of the world but have been removed from the U.S. market due to small, but statistically significant, increases in PSA and hematocrit.8
Some limitations exist with use of transdermal patches. The frequency of local irritation with these patches is significant. Up to two-thirds of patients report this adverse effect, and up to a 60% discontinuation rate has been described related to skin irriation.8 Headache, depression, rash, and gastrointestinal bleeding have been reported as well.1
Similar to testosterone gels, patches have proven to be efficacious in improving sexual function, body composition, and bone density. Because testosterone patches also avoid first-pass metabolism, this delivery form has not been associated with hepatic toxicity.2
Transbuccal System: One buccal delivery system of testosterone exists in the U.S. market. The system labeled Striant includes a 30-mg buccal tablet administered to the inner cheek or gum surface near the incisors. This tablet should be administered twice daily, releasing testosterone in a pulsatile manner similar to endogenous testosterone production. Buccal absorption occurs quickly, resulting in steady-state levels after the second dose. Efficacy trials have focused on improvement in sexual function and have demonstrated improvement compared to placebo in this area.8
Limitations to the use of this system exist and include local irritations of the mouth and gum, bitter taste, dry mouth, stomatitis, and anxiety. In addition, buccal tablets are often difficult to keep in place and require adherence to twice-daily administration. There is some concern of secondary exposure in saliva transfer with these agents.1
Intramuscular Injections: Intramuscular (IM) administration of testosterone involves injection of the testosterone esters, testosterone enanthate (Delatestryl) and testosterone cypionate (Depo-Testosterone). These have been recommended due to their lipophilicity, resulting in the storage and gradual release from the oil-based vehicle in which they are administered, and thus limiting the need for frequent injections. Effects of injectable testosterone are well established and have demonstrated benefits in improving sexual function, body composition, and mood.8 In comparison to other testosterone preparations, these agents are biologically effective at achieving normal virilization in all hypogonadal men.1
Recommended dosing regimens include 75 to 100 mg IM weekly or 150 to 200 mg IM every 2 weeks.7 Injectable testosterone is often the most cost-effective form of testosterone available, with each dose ranging in cost from $0.21 to $3.34.8
Limitations with IM injection of testosterone are most commonly injection site reactions. Up to 33% of men reported at least one local reaction, though discontinue rates did not correlate with number reported.8 In addition, due to the viscous nature of the vehicle in which testosterone is delivered and the muscularity of the injection site, a long (1.5 inch), large-gauge needle (20G) is required to administer the drug, which can be a barrier to some. Also, because of variability in absorption between patients, some may experience fluctuations in energy, mood, and libido due to the extended dosing interval.1 Gynecomastia has been reported in up to one-third of patients using injectable testosterone and usually presents within the first few days of therapy, when testosterone levels are at their highest.8 This complication is occasionally irreversible despite discontinuation of the product.
Subcutaneous Testosterone Implants: Testopel testosterone implants provide a depot preparation with a length of activity longer than that available with injections of testosterone. These implantable pellets help maintain eugonadal testosterone levels for months. The pellets dissolve slowly after subdermal implantation and have been reported in clinical trials to maintain normal serum testosterone levels up to 246 days after implantation.8 Adverse effects such as pellet extrusion, infection, and fibrosis have been reported.8 Implantation must be done in a specialist’s office, and the pellets can be implanted into the lower abdomen, deltoid, proximal thigh, or buttocks.
Human Chorionic Gonadotropin (hCG): As discussed previously, if induction of spermatogenesis is desired, TRT is often not effective. Consequently, administration of gonadotropins in an attempt to increase secretion of GnRH is recommended.9 The first agent administered is hCG. FSH is added once a plateau in response to hCG is determined.8 Gynecomastia is the most common adverse effected noted with these therapies.8
Monitoring and Safety
When starting testosterone therapy, the patient should be screened for conditions that could be exacerbated with supplementation. Screening prior to and during use ensures patient safety. Because risk of benign prostatic hyperplasia and prostate cancer is believed to be increased with testosterone therapy,10 patients must have a baseline PSA test performed with a digital rectal exam. The PSA level should be monitored during treatment, and the patient should be referred to a urologist if abnormalities are detected. Patients with a known history of breast cancer should also avoid testosterone therapy to prevent risk of recurrence.1,7,11
Erythrocytosis is a known adverse effect caused by excessive levels of testosterone; thus, a complete blood count (CBC) should be obtained at baseline and monitored thereafter.1,7 In addition, sleep apnea can be made worse with testosterone therapy. This condition may require referral for polysomnography and monitoring for daytime sleepiness and reported apneic episodes. Male pattern baldness and gynecomastia may occur in patients using testosterone therapy, both of which should be monitored as therapy continues. Additionally, acne and oily skin have been reported with testosterone administration.1,2,7,8
Conclusion
Pharmacologic management of males with hypogonadism can significantly improve their clinical symptoms, resulting in improved quality of life. There are a number of risks involved with therapy; however, the benefits of using TRT for hypogonadism can include increases in sexual function and improved mood, body composition, and bone density. Pharmacists play an essential role in ensuring that patients understand the administration of these therapies and are aware of the potential complications that can occur with their use.
REFERENCES
1. Snyder PJ, Matsumoto AM, Martin KA. Testos-terone treatment of male hypogonadism. UpToDate. www.uptodate.com. Updated September 30, 2009. Accessed January 3, 2010.
2. Beg S, Al-Khoury L, Cunningham GR. Testosterone replacement in men. Curr Opin Endocrinol Diabetes Obes. 2008;15:364-370.
3. Seftel AD. Male hypogonadism. Part I: Epidemiology of hypogonadism. Int J Impot Res. 2006;18:115-120.
4. Snyder PJ, Matsumoto AM, Martin KA. UpToDate. Causes of primary hypogonadism in males. www.uptodate.com. Updated September 30, 2009. Accessed January 3, 2010.
5. Snyder PJ, Matsumoto AM, Martin KA. Causes of secondary hypogonadism in males. UpToDate. www.uptodate.com. Updated September 30, 2009. Accessed January 3, 2010.
6. Snyder PJ, Matsumoto AM, Martin KA, et al. Clinical features and diagnosis of male hypogonadism. UpToDate. www.uptodate.com. Updated September 30, 2009. Accessed January 3, 2010.
7. Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in adult men with androgen deficiency syndromes: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2006;91:1995-2010.
8. Seftel A. Testosterone replacement therapy for male hypogonadism: part III. Pharmacologic and clinical profiles, monitoring, safety issues, and potential future agents. Int J Impot Res. 2007;19:2-24.
9. Layman LC. Hypogonadotropic hypogonadism. Endocrinol Metab Clin N Am. 2007;36:283-296.
10. Shabsigh R, Crawford ED, Nehra A, Slawin KM. Testosterone therapy in hypogonadal men and potential prostate cancer risk: a systematic review. Int J Impot Res. 2009;21:9-23.
11. Marks L, Mazer N, Mostaghel E, et al. Effect of testosterone replacement therapy on prostate tissue in men with late-onset hypogonadism: a randomized controlled trial. JAMA. 2006;296:2351-2361.
12. Testopel (testosterone pellets) package insert. Rye, NY: Bartor Pharmacal Co., Inc; April 1992.





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 