Raj W. Duggal, PharmD
PGY2 Oncology Pharmacy Resident
UC Health–University Hospital
Cincinnati, Ohio
Nicole J. Harger, PharmD, BCPS
Clinical Pharmacy Specialist, Emergency Medicine
UC Health–University Hospital
Cincinnati, Ohio
2/18/2011
US Pharm. 2010;36(2):HS11-HS16.
Tissue plasminogen activator (t-PA) is a natural peptide that initiates fibrinolysis by binding to fibrin in a thrombus and converting the trapped plasminogen to plasmin. Activity results in the breakdown of fibrin, fibrinogen, and other procoagulant proteins.1Administration of recombinant t-PA in the setting of an acute thromboembolic event will result in clot dissolution, reperfusion of organs, and restoration of blood flow to tissues.2
Acute ischemic stroke, acute myocardial infarction, and pulmonary embolism are life-threatening conditions that, without immediate treatment, may lead to serious complications including organ failure and death. These conditions may result when an acute thromboembolism decreases blood flow and oxygen delivery to organs. Administering thrombolytics, such as t-PA, to reverse underlying blockage can limit tissue damage and enhance patient outcomes.1,3-5
Thrombolytics are utilized regularly in emergency departments (EDs) for management of acute, life-threatening thromboses. Since there is a significant bleeding risk associated with therapy, patients must meet specific criteria for safe and effective use of these medications.1 Despite these criteria, inconsistencies in patient eligibility, dosing, and administration may be more common than realized.
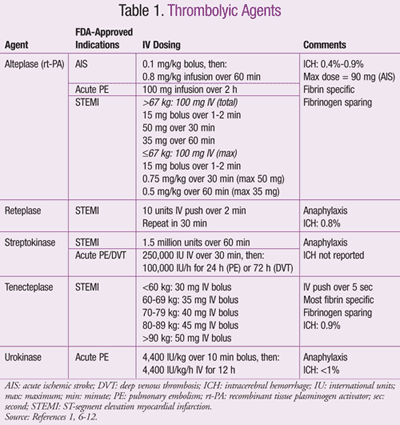
Available Thrombolytic Agents
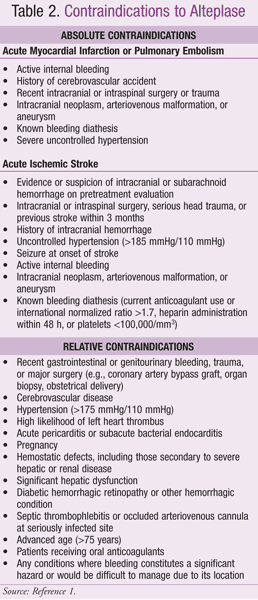
Commercially available thrombolytics with FDA-approved doses are included in TABLE 1.1,6-12 Alteplase (recombinant t-PA) is utilized most frequently in many EDs, as it has multiple indications with a low risk of anaphylactic reaction. Contraindications to alteplase are listed in TABLE 2.1
Acute Ischemic Stroke
The American College of Chest Physicians 2008 CHEST guidelines published recommendations for the use of thrombolytics in acute ischemic stroke (AIS).6 These recommendations are based on a pivotal trial by the National Institute of Neurological Disorders and Stroke (NINDS) evaluating the efficacy of alteplase versus placebo in patients presenting within 3 hours of AIS symptom onset. The results indicate that alteplase administration within 3 hours significantly decreases neurologic deficits at 90 days compared to placebo despite an increase in intracranial hemorrhages.3,6
In the ECASS III trial, Hacke et al reviewed the safety and efficacy of alteplase infusions versus placebo in the treatment of AIS up to 4.5 hours after symptom onset.5 Even with an increase in hemorrhagic transformations, 90-day disability is significantly decreased when alteplase is administered an average of 4 hours after symptom onset.5
Currently, additional evaluations are being conducted to determine alternative uses of t-PA within the ED for AIS management. The ongoing phase-II Combination of Rt-PA and Eptifibatide to Treat Acute Ischemic Stroke (CLEAR-ER) study is comparing the additional benefit of combining recombinant t-PA with eptifibatide in the management of an AIS to provide quicker and more complete breakdown of thromboses.13
This research stems from the Combined Approach to Lysis Utilizing Eptifibatide and rt-PA in Acute Ischemic Stroke (CLEAR) study by Pancioli et al, where the authors evaluated the safety of combining eptifibatide and rt-PA during an AIS.14 Ninety-four patients were enrolled in the original CLEAR trial, which demonstrated that t-PA and eptifibatide may safely be administered in AIS patients.14 The CLEAR-ER study was developed to further investigate the clinical and safety outcomes by monitoring for the development of an intracranial hemorrhage within 36 hours following the administration as well as changes in each individual’s Modified Rankin Score within 90 days.13
Intra-arterial administration of recombinant t-PA and extending the time interval postsymptom onset to 9 hours are also currently under evaluation.15,16
Desmoteplase, a derivative from bat saliva, is a novel thrombolytic agent being evaluated in phase II/III studies for AIS. Desmoteplase has high fibrin specificity, no activation by beta-amyloid, and minimal risk of the neurotoxicity seen with alteplase.17Conflicting evidence has been published surrounding efficacy of desmoteplase in the DIAS, DIAS-2, and DEDAS studies.17Ongoing evaluations of desmoteplase versus placebo continue in the phase III DIAS-3 study.18
Acute Myocardial Infarction
The management of an acute coronary syndrome (ACS) is a protocol-driven process for most institutions, and thrombolytics have a defined role for use in the ED. Thrombolytics do not play a role in the management of unstable angina or non-ST-segment elevation myocardial infarctions (NSTEMIs) but may be used to manage ST-segment elevation myocardial infarctions (STEMIs) in specific situations.7,8 While the standard of care treatment for STEMI remains percutaneous coronary intervention (PCI), thrombolytic therapy is considered an alternative for patients unable to undergo PCI due to patient variables or lack of availability.8
Many institutions are equipped to readily perform PCI or are able to quickly transfer patients to a nearby hospital where PCI is available. If this is not the case, thrombolytic therapy is employed. Alteplase and tenecteplase have evidence advocating use within 6 hours of symptom onset, while streptokinase and reteplase may be considered within 12 hours of symptom onset.7,8
Limited research is evaluating additional indications for thrombolytic therapy during ACS. In the ongoing Strategic Reperfusion Early After Myocardial Infarction (STREAM) study, investigators are evaluating the efficacy of combining thrombolytic, antiplatelet, antithrombotic, and PCI therapies. Interventions include tenecteplase plus clopidogrel and enoxaparin therapy prior to PCI versus standard PCI within 3 hours of symptom onset to evaluate for 30-day clinical outcomes.19
Acute Pulmonary Embolism
Thrombolytics have demonstrated efficacy in treating hemodynamically stable and unstable patients with massive and submassive pulmonary embolism (PE).20 A massive PE is defined as the presence of respiratory collapse/hypotension, unexplained hypoxia, engorged neck veins, and right ventricular gallop.21
If left untreated, a PE may continue to expand, increasing pulmonary vascular resistance to cardiac muscle. This could result in suppressed function and lead to cardiac arrest. Twenty percent of patients presenting in cardiac arrest are in pulseless electrical activity (PEA).22 PEA arrest is the result of a major cardiovascular, respiratory, or metabolic derangement where some forward blood flow may occur. Situations that cause sudden changes in preload, afterload, or contractility often result in PEA. One potential cause of cardiovascular compromise is occlusion of normal circulation from a massive PE. Approximately 70% of people experiencing an out-of-hospital arrest have underlying acute myocardial infarction or PE and could benefit from thrombolytic therapy.23
In an evaluation of 256 hemodynamically stable patients with poor prognostic factors, patients were randomized to receive alteplase 100 mg (10 mg bolus followed by 90 mg over 2 hours) or placebo in addition to a standard heparin infusion. Similar mortality was reported between the two groups, but there was a significantly higher need to escalate therapy in the placebo arm and a significantly higher 30-day event-free survival in the alteplase arm.20
A recent trial compared the administration of alteplase versus placebo during cardiopulmonary resuscitation (CPR) for patients presenting to the ED in cardiac arrest with PEA. Of the 233 patients enrolled in the study, one patient treated with alteplase survived to hospital discharge, while no patients receiving placebo survived.22 As is the case in many cardiac arrest trials, there was a high (63%) onsite mortality among all patients within the study.22 Additionally, 2% of alteplase-treated patients in this trial experienced an intracranial hemorrhage. While these results do not indicate significant improvement in clinical outcomes, the authors conclude that alteplase should still be considered in patients in PEA arrest at high risk for embolism as a primary cause.22 While not an FDA-approved indication, use of t-PA in cardiac arrest with PEA mechanistically makes sense, since the arrest could be precipitated by an occluding PE.22,23
The British Thoracic Society published guidelines in 2003 addressing t-PA dosing for patients presenting with an acute PE and recommended alteplase 50-mg IV bolus in patients with massive PE whose cardiac arrest is imminent.21 These guidelines do not recommend routinely utilizing thrombolytics for submassive PE, as there is no significant survival advantage and there are increased hemorrhagic complications.21
Currently a few ongoing studies are investigating the role of thrombolytics in patients for the management of a PE. The Pulmonary Embolism Thrombolysis (PEITHO) study is evaluating the impact of tenecteplase in addition to standard anticoagulation in patients with a submassive PE for the composite end point of mortality and hemodynamic collapse at 7 days.24 The Ultrasound Accelerated Thrombolysis of Pulmonary Embolism (ULTIMA) study is evaluating the utility of determining the ratio of the right ventricular end diastolic diameter to the left ventricular end diastolic diameter as a risk factor for complications and the benefit of ultrasound-guided low-dose (<20 mg recombinant t-PA) thrombolytics in patients with a ratio greater than or equal to 1.25
Clinical Experiences
Thrombolytics introduce potential life-threatening complications to those receiving therapy. Due to this high risk, medication errors may occur during administration of thrombolytics in the ED related to both ordering and administering the medication, potentially leading to negative outcomes. Unpublished data from an internal, single-institution review of patients receiving t-PA treatment for all indications estimated that approximately 70% of patients receiving therapeutic thrombolytic therapy received an appropriate dose given their indication and weight.26 Limited documentation prevented verification of appropriate doses in 20% of patients evaluated.FIGURE 1 illustrates the distribution of doses determined to be appropriate or inappropriate given the indication and patient-specific factors from this internal review.26
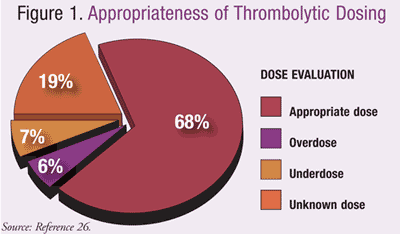
Using estimated weights to dose thrombolytics is of concern, as patients are at risk of receiving excessive or suboptimal doses. Verifying accurate weights may be difficult, as many patients are not stable enough to provide the information or stand on traditional scales. Bed scales in the ED may enhance appropriate dosing and may maximize therapeutic benefit while limiting adverse effects.
Pharmacy involvement in the ED may significantly decrease the number of errors in ordering, compounding, and administering thrombolytics. Prominent clinical pharmacy services within the ED may help minimize life-threatening errors. Additionally, preprinted orders for thrombolytic therapy may standardize ordering and enhance accurate documentation for the use of high-alert medications.
REFERENCES
1. Activase (alteplase) package insert. South San Francisco, CA: Genentech, Inc; 2002.
2. Marin F, Gonzales-Conejero R, Capranzano P, et al. Pharmacogenetics in cardiovascular antithrombotic therapy.J Am Coll Cardiol. 2009;54:1041-1057.
3. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581-1587.
4. Thabut G, Thabut D, Myers RP, et al. Thrombolytic therapy of pulmonary embolism: a meta-analysis. J Am Coll Cardiol. 2002;40:1660-1667.
5. Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317-1329.
6. Albers GW, Amarenco P, Easton JD, et al. Antithrombotic and thrombolytic therapy for ischemic stroke: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th ed). Chest. 2008;133(suppl 6):630S-669S.
7. Harrington RA, Becker RC, Cannon CP, et al. Antithrombotic therapy for non-ST-segment elevation acute coronary syndromes: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th ed).Chest. 2008;133(suppl 6):670S-707S.
8. Goodman SG, Menon V, Cannon CP, et al. Acute ST-segment elevation myocardial infarction: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines(8th ed). Chest. 2008;133 (suppl 6):708S-775S.
9. Retavase (reteplase) package insert. McPherson, KS: Hospira, Inc; 2006.
10. TNKase (tenecteplase) package insert. South San Francisco, CA: Genentech, Inc; 2008.
11. Kinlytic (urokinase) package insert. Tucson, AZ: ImaRx Therapeutics Inc; 2008.
12. Kearon C, Kahn SR, Agnelli G, et al. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th ed). Chest. 2008;133 (suppl 6):454S-545S.
13. University of Cincinnati; National Institute of Neurological Disorders and Stroke (NINDS). Study of the Combination of Rt-PA and Eptifibatide to Treat Acute Ischemic Stroke (CLEAR-ER). www.clinicaltrials.gov/ct2/show/NCT00894803. Accessed December 14, 2010.
14. Pancioli AM, Broderick J, Brott T, et al. The combination approach to lysis utilizing eptifibitide and rt-pa in acute ischemic stroke. Stroke. 2008;39:3268-3276.
15. University of Cincinnati; National Institute of Neurological Disorders and Strokes (NINDS); Medical University of South Carolina; University of Calgary. Interventional Management of Stroke (IMS) III Trial (IMS III). www.clinicaltrials.gov/ct2/ show/NCT00359424. Accessed December 14, 2010.
16. National Stroke Research Institute, Australia; Commonwealth Scientific & Industry Research Organisation; Brain Research Institute; University of Melbourne; Melbourne Health. Extending the Time for Thrombolysis in Emergency Neurological Deficits (EXTEND). www.clinicaltrials.gov/ct2/ show/NCT00887328. Accessed December 14, 2010.
17. Hacke W, Furlan AJ, Al-Rawi Y, et al. Intravenous desmoteplase in patients with acute ischemic stroke selected by MRI reperfusion-diffusion weighted imaging or perfusion CT (DIAS-2): a prospective, randomised, double-blind, placebo-controlled study. Lancet Neurol. 2009;8:141-150.
18. H. Lundbeck A/S. Efficacy and Safety Study of Desmoteplase to Treat Acute Ischemic Stroke (DIAS-3). www.clinicaltrials.gov/ct2/ show/NCT00790920. Accessed January 17, 2011.
19. Armstrong PW, Gershlick A, Goldstein P, et al. The Strategic Reperfusion Early After Myocardial Infarction (STREAM) study. Am Heart J. 2010;160:30-35.
20. Konstantinides S, Geibel A, Heusel G, et al. Heparin plus alteplase compared with heparin alone in patients with submassive pulmonary embolism. N Engl J Med. 2002;347:1143-1150.
21. British Thoracic Society Guideline Development Group. British Thoracic Society guidelines for the management of suspected acute pulmonary embolism. Thorax. 2003;58:470-484.
22. Abu-Laban RB, Christenson JM, Innes GD, et al. Tissue plasminogen activator in cardiac arrest with pulse-less electrical activity. N Engl J Med. 2002;346:1522-1528.
23. Bottiger BW, Arntz HR, Chamberlain DA, et al. Thrombolysis during resuscitation for out-of-hospital cardiac arrest. N Engl J Med. 2008;359:2651-2662.
24. Assistance Publique-Hôpitaux de Paris; German Federal Ministry of Education and Research; Boehringer Ingelheim Pharmaceuticals. PEITHO Pulmonary Embolism Thrombolysis study. www.clinicaltrials.gov/ct2/show/NCT00639743. Accessed December 14, 2010.
25. EKOS Corporation. Ultrasound Accelerated Thrombolysis of Pulmonary Embolism (ULTIMA). www.clinicaltrials.gov/ct2/ show/NCT01166997. Accessed December 14, 2010.
26. The safe and appropriate use of t-PA in the emergency department: a retrospective review of prescribed thrombolytics. Cincinnati, OH: UC Health—University Hospital; 2010. Data on file.





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 