最近剛好遇到一個問題:有個病人體重299公斤(無誤),他要使用enoxaparin....問題來了喔,大家都知道,這個藥1mg/kg q12h(對正常腎功能的病患而言),而且沒有什麼很有效的antidote....所以,大家覺得這劑量怎麼給好?
事實上,也有前輩寫過相關文章:
[臨床藥學]當enoxaparin遇到肥胖病人 (Weight-Based Dosing of Enoxaparin in Obese Patients)
Clinical Pharmacology & Therapeutics 82, 505-508 (November 2007)
The global epidemic of obesity has led to an increased prevalence of chronic diseases and need for pharmacological intervention. However, little is known about the influence of obesity on the drug- exposure profile, resulting in few clear dosing guidelines for the obese. Here we present a semi-mechanistic model for lean body weight (LBW) that we believe is sufficiently robust to quantify the influence of body composition on drug clearance, and is therefore an ideal metric for adjusting chronic dosing in the obese.
Obesity has reached epidemic propor- tions worldwide, and the obese can no longer be considered a minority demo- graphic.1 Despite increased pharmaco- therapy among obese patients, there is a paucity of dosing guidelines for this pop- ulation. This could be partly attributed to insufficient knowledge about phar- macokinetic parameters as a function of body composition due to the exclusion of obese subjects from clinical trials (in which body composition is defined as the differentiation of lean tissue from body fat in an individual). In addition, there has been no suitable size descriptor for dose adjustments across a wide range of body compositions.2 It should be noted that dose adjustments referred to in this commentary pertain to maintenance doses, not loading doses.
This commentary aims to (i) present a recent derivation of a size metric, lean body weight (LBW), which takes into account changes in body composition that occur with obesity, and (ii) pro- pose a hypothesis that LBW is sufficient to explain the influence of body com- position on clearance and can therefore adequately predict drug exposure in the obese. We do this not only to highlight the complexity of designing appropri- ate studies to investigate the impact of obesity on drug clearance, but also to formally recommend that our hypoth- esis be the subject of future testing. It is our belief that dose individualization can be significantly improved by under- standing the quantitative relationship between body composition and drug clearance. We also believe that this relationship should be described by a mechanistically derived dosing scalar, thereby enabling it to provide quanti- tative predictions about the impact of body composition on the drug-expo- sure profile. This goal is in line with the Food and Drug Administration’s (FDA) Critical Path Initiative,3 which seeks to improve understanding of the expo- sure–response relationship.
In clinical practice, conventional methods of dose adjustment via weight- based regimens, i.e., milligram per kilo- gram, assume that biological functions are directly proportional to total body weight (WT). However, 99% of the body’s metabolic processes (including clearance) take place within lean tissues.4 We contend that using WT to calculate maintenance doses for obese patients is scientifically unsound. The obese have a lower LBW/WT ratio overall,5 although their total LBW is greater than that of normal-weight individuals. Because WT represents the integral of body compo- nents, it is too simplified for describing changes in body composition that occur with obesity. We believe that a two-com- partment model, with LBW as Compart- ment 1, is the simplest model needed to adequately describe body composition.
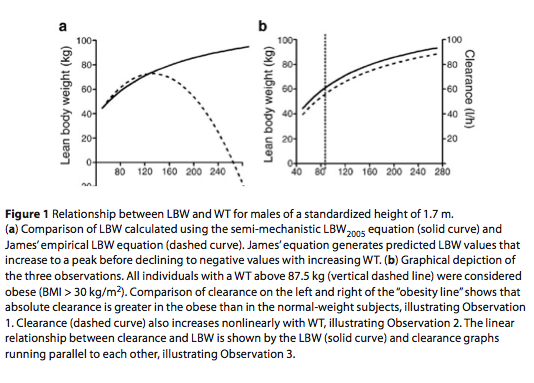
LBW represents the sum of cellular mass and nonfatty intercellular connective tissue, such as bone (excluding fatty mar- row), tendons, ligaments, and basement membranes.4 It should be noted that indi- viduals used in the derivation of James’ LBW equations in 1976 (maximum body mass index (BMI) = 43 kg/m2; maximum WT = 122 kg)6 weighed considerably less than obese patients commonly found in the clinic today (maximum BMI = 100 kg/m2; maximum WT = 273 kg).7 This underrepresentation of obese subjects in James’ population (only 9.2% of study subjects had a BMI > 30 kg/m2) resulted in LBW equations that provided biologi- cally implausible estimates of LBW in the form of negative values (Figure 1a).8
To overcome the limitations of James’ empirical LBW equations, a semi-mech- anistic model for LBW, based on bio- impedance, was developed in 2005 in a population with wide-ranging WTs that are representative of the current popula- tion. These equations are shown below; further details on their derivation are available in Janmahasatian et al.9:
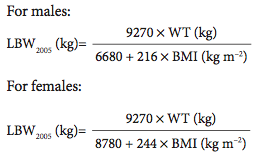
One key advantage of the LBW2005 model, apart from agreeing well with James’ LBW equations over normal ranges of height and WT, is that the esti- mate of LBW2005 never declines as WT increases (Figure 1a). These equations also have good predictive properties when compared with LBW derived from dual- energy X-ray absorptiometry (DXA),9 a reference method for LBW estimation.
With LBW2005, we now have a robust size descriptor capable of quantifying changes in hepatic and renal clearance for individuals of a wide range of body com- positions, which sets the stage for a con- ceptual shift in the way we perceive the relationship between body composition and clearance. This relationship translates into the following hypothesized observa- tions, which are graphically depicted in Figure 1b:
Observation 1: Absolute clearance is greater in obese individuals.
Observation 2: Clearance increases nonlinearly with WT.
Observation 3: Clearance correlates linearly with LBW.
Our proposal that LBW and clearance are linearly related is based on mecha- nistic principles derived from prior biological knowledge and differs from earlier investigations that drew empiri- cal relationships between body compo- sition and clearance. It is probable that technical challenges in measuring and relating pharmacokinetic parameters to LBW prevented the use of a mechanistic
approach when performing such stud- ies. Although prior investigators alluded to the possibility that excess adipose tis- sue influenced pharmacokinetic param- eters in the obese, we surmise that their progress was hindered by the use of inap- propriate size descriptors such as ideal body weight (IBW).10 IBW is a function of height and sex only, and does not take into account differences in body com- position. Use of IBW as a surrogate for lean mass is inherently flawed, because it assumes that individuals of the same sex and height have exactly the same ideal (and presumably lean) mass. Understand- ably, this approach confounds the ability to identify the true impact of obesity and body composition on drug clearance.
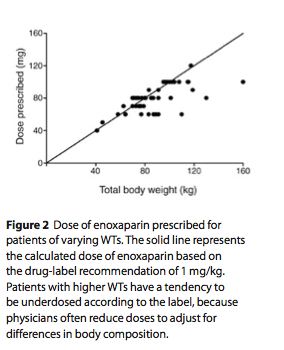
Given the availability of methods to precisely determine LBW (e.g., DXA, bioimpedance) and an improved under- standing of how LBW changes across individuals of varying body composition (with the increased prevalence of obes- ity), we recommend that investigators design and analyze dose-exposure trials with clearance expressed as a function of LBW. Results from such studies can also be used to predict drug exposure in the obese, even if they were excluded from the studies. This provides a distinct advantage over empirical dosing strategies based on WT, which are likely to result in overdose when extended to the obese. In fact, phy- sicians often attempt to adjust for altered body composition in the obese by arbi- trarily reducing weight-based drug doses. A good example is the drug enoxaparin, which has a labeled dose of 1 mg/kg. An observational study (n = 50) has shown that physicians do not prescribe 1 mg/kg in the obese (probably because of the risk
of adverse bleeding events) and arbitrar- ily reduce the dose to account for body composition (Figure 2).11
Having recognized the shortcomings of using empirical methodologies dur- ing the drug-development process, the FDA launched the Critical Path Initia- tive in 2004 to promote the concept that scientific tools for predicting and evalu- ating drug safety and efficacy should be developed mechanistically.3 Although empirical methodologies can ensure that the drug is right for its intended thera- peutic purposes by confirming its aver- age effectiveness on a population level, ensuring the right dose on an individu- alized basis can be achieved only by using mechanistic approaches that build on improved insights into the influence of patient variables on the dose exposure– response relationship.12
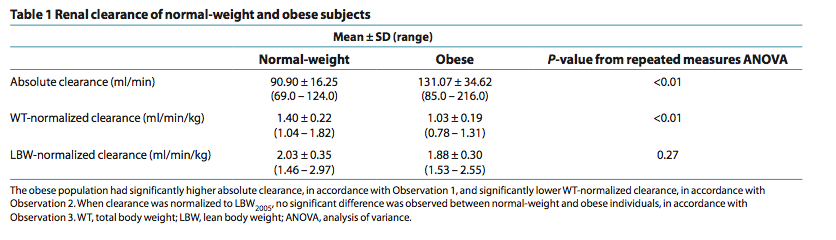
Given that the concept of relating drug clearance to LBW rather than WT is rela- tively new, it is understandable that prior studies were not designed specifically to test this hypothesis. We have reanalyzed renal clearance data from a previous study using LBW2005 in which absolute clearance, as well as clearance values normalized by WT and LBW2005 for normal-weight and obese subjects, were compared using repeated-measures analy- sis of variance (Table 1) (unpublished data from S. Janmahasatian, S.B. Duffull, A. Chagnac, C.M.J. Kirkpatrick, and B. Green). The results of this study concur with our hypothesis that drug clearance for individuals of varying heights and WTs is the same after adjusting for body composition. They also imply that LBW is a useful dosing scalar that is transportable across all body compositions.
Although our hypothesis is novel, other investigators have similarly recognized the limitations of using conventional dos- ing scalars in obese patients. Shibutani et al.13 observed that the absolute clear- ance for a hepatically cleared drug (fen- tanyl) was significantly higher in obese patients and that clearance increased nonlinearly with WT. They solved for their observations by deriving a size metric termed “pharmacokinetic mass” that increased linearly with clearance. We have compared their empirically derived “pharmacokinetic mass” with our semi- mechanistic LBW model for subjects with WTs and heights from 35 to 165 kg and 1.3 to 1.95 m, respectively, and found a close correlation between the two size descriptors (R2 = 0.92 for males and R2 = 0.94 for females). Their results agree with Observations 1 and 2 and indirectly accord with Observation 3, thus provid- ing additional evidence in support of our hypothesis.
We have also reviewed other stud- ies examining the influence of obesity on drug clearance published between 1978 and 2004 (Supplementary Table S1 online). A complete reanalysis of the data is not possible because of unavail- able published individual data and lack of studies specifically designed to test our hypothesis. Of the 72 studies, only 5 used LBW or a similar size metric (“pharma- cokinetic mass”). The remainder defined obesity as a function of IBW or BMI. The inherent flaws of IBW as a surrogate for lean mass, as discussed above, only serve to confound the results. BMI is also an unsuitable body composition metric, because it does not distinguish between excess fat and a larger muscle/bone mass.
Author-reported conclusions from stud- ies using IBW or BMI should therefore be interpreted with caution.
Nevertheless, these studies provide empirical support that body composition is sufficient to explain interindividual variability in drug clearance. The abso- lute clearance and clearance normalized by WT or LBW reported in the studies, where available, were tabulated. Studies that showed any of the three observations described above were deemed to support the hypothesis, and studies that showed nonsignificant results or were underpow- ered to show a difference were considered to fail to disagree with the hypothesis. Studies that showed any one of the three observations but showed the opposite for any of the other two conditions were regarded as disagreeing with the hypoth- esis. Of the 80 investigations carried out (some studies investigated more than one drug), 21.2% agreed with the hypothesis and 65% failed to disagree. An additional 10% disagreed but could be explained by confounders, leaving a 3.8% error rate, which is less than the commonly accepted alpha error of 5%. Despite the fact that these studies were not designed to directly address the hypothesis, the majority failed to disagree with the hypothesis, thus sup- plying empirical evidence for our pro- posed relationship between clearance and body composition.
We hope that future studies will place greater emphasis on reporting phar- macokinetic results that mechanisti- cally quantify the relationship between clearance and body composition as a function of LBW, even if obese subjects are excluded from these studies. This approach will enable quantitative predic- tions about the dose exposure–response relationship in the obese, thereby opti- mizing pharmacotherapy. To ensure that future studies can evaluate our hypothe- sis, we suggest that investigators consider incorporating the following proposals into their trial designs:
1. Body composition should be meas- ured as a function of LBW rather than IBW or BMI. This avoids the limitations of using IBW and BMI (whose flaws have been described earlier) to describe body com- position. LBW should be estimated using derived equations (e.g., Janmahasatian et
al.9), DXA, or other established methods. 2. If the objective is to determine whether differences in drug clearance exist between obese and normal-weight subjects using null-hypothesis testing, the two groups must not be matched for any variable (e.g., LBW) that correlates
with clearance.
3. Studies should be designed from
a “learning” perspective,14 with a con- tinuum of subject size ranging from normal-weight to obese and including a wide range of (and preferably stratified for) LBWs. Analysis should be based on regression techniques, allowing quan- tification of clearance as a function of LBW. This differs from the null-hypoth- esis testing approach, which provides only a yes–no answer to the question of interest.
The “one size fits all” concept of dosing is gradually being replaced by strategies aimed at delivering “personalized medi- cine” to patients. These have the potential to improve drug efficacy and minimize adverse events, hence optimizing patient care and reducing health-care costs from suboptimal dosing. In addition, the use of appropriate dosing scalars is likely to accelerate the approval of new drugs by ensuring drug safety across different patient subpopulations. We believe that using LBW as a dosing scalar is vital to achieving these goals, although we acknowledge that our proposed rela- tionship between body composition and clearance accounts for only a portion of the predictable aspects of interindividual variability in drug clearance. Regretta- bly, despite an improved understanding of the physiologic changes associated with obesity and its rising prevalence worldwide, the FDA has yet to recog- nize obese patients as a separate entity with characteristics that differ from the rest of the population. A simple solution to overcome this lack of special dosing instructions for the obese is to design and analyze dose exposure–response studies using LBW as the size metric on clear- ance, and to report dosing recommenda- tions as a function of LBW in drug labels. Furthermore, because LBW performs equally well in normal-weight and obese individuals, we postulate that results of dose-ranging studies using a milligram-
per-kilogram-of-LBW approach in nor- mal-weight subjects can be extrapolated to the obese population despite their exclusion from clinical trials. This strat- egy is timely, given the increased inci- dence of obesity-related comorbidities requiring pharmacotherapy, and provides physicians with opportunities to make meaningful clinical decisions about dos- ing in obesity.
監測Anti-Xa應該是比較正確的做法,不過不確定血汗醫院可不可以做到。
另外,雖然不知道應該要打幾折,不過可以肯定的是,越胖的病人,應該要打的折扣越多^^,從低劑量開始使用,然後慢慢的調升我想應該是比較安全的。
順便來介紹一下關於anti-Xa的測定範圍:Peak levels are monitored 4 hours after subcutaneous injection. Although therapeutic ranges are not well established, target level of 0.6 to 1 IU/mL for twice daily dosing and 1 to 2 IU/mL for once daily dosing have been suggested--CCIS
老實說如果真的沒有把握,其實也許可以考慮使用UFH....這樣有幾點好處:
1.可以IV給藥。
2.半衰期短(1.5 hr (anticoagulation effect half-life--CCIS))
3.可以用protamine當antidote.
唯一要注意的是,可能會產生HIT,這一點必須要特別留意。不過不論怎樣,目前大多數的guildline都是建議使用LMWH就是了。
(以上內容感謝彰基藥師提供)
其他抗凝血的藥物用法:[US pharmacist]The Safe and Appropriate Use of Thrombolytics in the Emergency Department






 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 