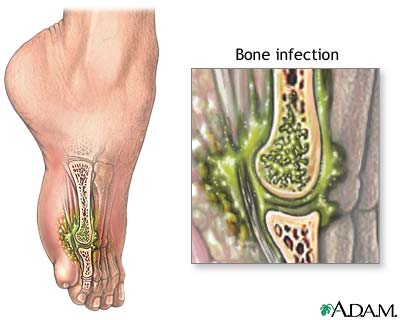
骨髓炎就是骨頭受到病菌的感染引起發炎反應(bone infection)。骨頭與身體其他的組織器官一樣,也是容易受到細菌的侵犯。造成骨髓炎的細菌以化膿性的細菌居多,結核菌、梅毒菌、黴菌與濾過性病毒均可以造成骨髓炎。病菌入侵骨頭之後會形成炎性反應,進一步造成膿瘍而破壞骨頭。病菌通常是經由血液循環到骨頭來,但是骨髓炎也可以是經由軟組織感染的直接擴散,或經由是穿刺傷入侵骨頭,像是開放性骨折(皮膚裂開使得骨頭與外界直接相通)。最常見的感染細菌是金黃葡萄球菌,其次是溶血性的鏈球菌。
之前也有介紹過:
Selection of antibiotics for treatment of acute osteomyelitis 急性骨髓炎的抗生素選擇
US Pharm. 2012;37(11):HS-2-HS-8.
ABSTRACT: Chronic infections of bone and joint usually occur in adults from trauma, contiguous soft-tissue infections, or orthopedic implants. In most cases Staphylococcus aureus is the pathogen, although other bacteria are also involved. Symptoms include pain, fever, wound drainage, and necrosis. Treatment options include surgical removal of dead tissues and orthopedic implants and prolonged (≥6 wk) antibiotic therapy. Antibacterial therapy administered parenterally or orally has produced similar therapeutic benefits. Current literature supports oral therapy as a viable option for long-term treatment of chronic osteomyelitis; however, sufficient data from well-designed comparative studies are lacking. Upon completion of these studies, concerns regarding dosing, duration, and combination oral therapy may be resolved.
Chronic osteomyelitis, a complicated infection of bones and joints, usually occurs in adults and produces symptoms after several weeks.1Unlike the acute form, which results from the hematogenous spread of bacteria, chronic osteomyelitis develops from fractures, open wounds, contiguous soft-tissue infections, and orthopedic implants.1 Patients with diabetes mellitus (DM) or peripheral vascular disease are prone to chronic osteomyelitis from infection of contiguous tissues. Symptoms include chronic pain, wound and sinus tract drainage, malaise, fever, and bone necrosis.1
In most cases of chronic osteomyelitis, Staphylococcus aureus is the causative organism.1 Chronic bone infection may also be caused byStaphylococcus epidermidis, Pseudomonas aeruginosa, Serratia marcescens, Escherichia coli, Streptococcus, and anaerobes.1Immunodeficient patients may develop bone and skeletal infections from fungal and mycobacterial species. It is important to note that 1) methicillin-resistant S aureus (MRSA) is becoming more prevalent in chronic osteomyelitis2 and 2) some causative organisms are capable of forming biofilms.3 Biofilm is an extracellular polymeric matrix that renders eradication of pathogenic bacteria by antimicrobial treatment virtually impossible.3 Detailed diagnostic procedures, however, are beyond the scope of this article.
Chronic osteomyelitis is diagnosed by laboratory findings (erythrocyte sedimentation rate, C-reactive protein), imaging (x-ray, MRI), and microbiological examination that includes antibiotic sensitivity of infected tissues.1
Disease Management
Chronic osteomyelitis management remains a major clinical challenge.1 Current treatment consists of surgery plus prolonged (≥6 wk) antibiotic therapy. Surgery is necessary to remove necrotic and devitalized bone and tissues and orthopedic devices.1 Despite these approaches, rate of treatment failure is high. Following initial success, the recurrence rate of chronic osteomyelitis at 1 year is 30%.4 The overall goal thus far has been to limit the spread of infection to adjacent healthy bones and tissues.
In the past, chronic osteomyelitis was treated parenterally. In recent years, however, oral therapy has been found to be therapeutically equivalent to parenteral treatment.5 Important factors in the consideration of oral administration include (1) excellent bone concentration; (2) activity against targeted organisms; (3) reduced incidence of resistance; (4) fewer adverse effects (AEs), even at high doses; and (5) cost-effectiveness. This article will discuss pharmacologic properties of orally active antibacterial agents and summarize clinical trials evaluating these agents’ efficacy and safety in chronic osteomyelitis.
Orally Active Antibacterial Agents
Fluoroquinolones (Ciprofloxacin, Ofloxacin, Levofloxacin): Fluoroquinolones are fluorinated analogues of nalidixic acid with a broad spectrum of antibacterial activity and excellent oral bioavailability.6 These agents inhibit DNA gyrase and topoisomerase IV, enzymes that are critical for DNA replication. Following absorption, fluoroquinolones are widely distributed in various tissues, including the bones, prostatic fluid, cerebrospinal fluid (CSF), bile macrophage, lungs, and kidneys.6
Other than moxifloxacin, which is excreted in the bile, all fluoroquinolones are excreted renally. Cations (iron, aluminum, calcium) chelate fluoroquinolones and reduce their absorption. Antacids and hematinics should not be given within 2 hours of fluoroquinolone administration. Fluoroquinolones inhibit metabolism of theophylline (a phosphodiesterase inhibitor used to treat asthma) and warfarin (a vitamin K inhibitor); hence, clinical monitoring may be required. AEs associated with fluoroquinolones include increased risk of tendon rupture and tendinitis (Black Box Warning), exacerbation of muscle weakness in patients with autoimmune disease, myasthenia gravis (Black Box Warning), gastrointestinal (GI) symptoms, photosensitivity, central nervous system effects (headache, dizziness, confusion, lightheadedness, hallucinations, delirium), and QT-interval prolongation resulting in torsades de pointes.6 Fluoroquinolones should be avoided in uncorrected hypokalemia and with other arrhythmogenic agents (e.g., class IA and III antiarrhythmics, erythromycin).
Rifampin: Widely used to treat tuberculosis, rifampin is a semisynthetic derivative of rifamycin SV.7 It inhibits bacterial DNA–dependent RNA polymerase, an enzyme that polymerizes bacterial RNA. Nuclear RNA polymerases of mammalian cells do not bind to rifampin and are unaffected by the drug. At high concentrations, however, rifampin can inhibit mitochondrial RNA synthesis in mammalian cells.7Rifampin is well absorbed orally and is widely distributed in different organs and fluids, including CSF. The parent drug undergoes deacetylation; this metabolite retains antibacterial properties. Rifampin is predominantly excreted in the bile. AEs associated with rifampin include elevated liver enzymes, flulike syndrome (fever, myalgia, chills, headache, hypotension, shock), and orange-colored urine, saliva, sweat, and other bodily fluids.7 Rifampin induces different CYP enzymes (CYP1A2, CYP2D6, CYP2C9, CYP2C19, and CYP3A4); therefore, it can reduce serum levels of drugs metabolized by these enzymes if administered concomitantly. Administration of an antacid with rifampin has been shown to reduce the concentration of rifampin and should be avoided.7
Linezolid: Linezolid is a synthetic, broad-spectrum antibacterial agent available in both oral and parenteral dosage forms. It binds to 23S rRNA of the 50S ribosomal subunit and prevents formation of 70S complex, which initiates bacterial protein synthesis.8 Following oral dosing, maximum plasma concentration is achieved within 1 to 2 hours, and bioavailability reaches approximately 100%. Time to reach maximum concentration may be delayed when linezolid is taken with a fatty meal. Linezolid is not an inducer or inhibitor of CYP enzymes.8It is a nonselective inhibitor of monoamine oxidase and can interact with adrenergic and serotonergic agents.8 The most common AEs associated with linezolid are headache, nausea, and vomiting. Thrombocytopenia can occur when linezolid is used in high doses for a prolonged period.8
Sulfamethoxazole-Trimethoprim (SMX-TMP): This combination is used to sequentially inhibit DNA synthesis in sensitive bacteria.9 SMX, a sulfonamide, inhibits dihydropteroate synthase, which is responsible for incorporating para-aminobenzoic acid into dihydropteroic acid; the latter is a precursor of tetrahydrofolate. TMP, a diaminopyrimidine, inhibits dihydrofolate reductase, which is responsible for converting dihydrofolate to tetrahydrofolate.9 Tetrahydrofolate is used for DNA synthesis. Mammalian cells cannot synthesize folic acid and hence are unaffected by these agents.
SMX-TMP is rapidly absorbed orally and is widely distributed in different organs. The drug combination is bound to plasma protein in variable concentrations.9 SMX is metabolized to an N4-acetyl derivative; TMP is metabolized to oxides and hydroxides. SMX-TMP is excreted renally. The most common AEs for SMX are hypersensitivity reactions (rashes, drug fever, exfoliative dermatitis, Stevens-Johnson syndrome) and hematologic disturbances (agranulocytosis, aplastic anemia).9 TMP can cause rash, glossitis, stomatitis, and GI disturbances. The combination product may cause thrombocytopenia.
Aminopenicillins (Ampicillin, Amoxicillin): Aminopenicillins are so named because of the presence of an amino group in the side chain.10 Like other penicillins, aminopenicillins are bactericidal agents and inhibit bacterial cell wall synthesis. Aminopenicillins inhibit transpeptidase, which is responsible for crosslinking of the bacterial cell wall.10 Crosslinking lends rigidity and structural support to the cell wall. Aminopenicillins can induce murein hydrolases, which destroy existing cell wall. Ampicillin and amoxicillin also can bind to intracellular penicillin-binding proteins and disrupt their functions.
Amoxicillin is given orally, whereas ampicillin is administered parenterally. Amoxicillin achieves higher plasma and tissue levels than ampicillin. Both agents are excreted renally. In addition to severe hypersensitivity reactions common to all penicillins, aminopenicillins can cause diarrhea and Clostridium difficile enterocolitis. A characteristic rash can develop when ampicillin is administered with allopurinol. Aminopenicillins interfere with the absorption of oral contraceptives.10 Agents known to interact with aminopenicillins are probenecid (promotes renal excretion of uric acid), aminoglycosides (physical interaction with aminopenicillins results in aminoglycoside inactivation), tetracyclines (cidal vs. static drug interaction reduces cidal penicillin’s effectiveness), and methotrexate (reduced methotrexate excretion with subsequent toxic reactions).
Beta-Lactamase Inhibitors (Sulbactam, Clavulanic Acid): These agents inhibit beta-lactamase, an enzyme produced by both gram-positive and gram-negative bacteria. Beta-lactamase destroys the beta-lactam ring of penicillin antibiotics, rendering them inactive.10 These inhibitors have minimal antibacterial activity, if any, but they are used to increase the antibacterial spectrum of aminopenicillins (ampicillin and amoxicillin) when used in combination.
Randomized Controlled Trials
Fluoroquinolones: Ciprofloxacin was compared with the standard parenteral regimen for osteomyelitis in a prospective, randomized trial.5Prior to the study, infective wounds were surgically debrided and metallic implants removed. Ciprofloxacin patients (n = 31) received 750 mg twice daily by mouth for an average of 56 days.5 The parenteral group (n = 28) received IV ceftazidime (a third-generation cephalosporin) or a nafcillin-amikacin (or another aminoglycoside) combination for a mean of 47 days. In the parenteral group, antibiotic dosing could be modified to avoid toxic reactions. The cure rate was 77% in the ciprofloxacin group, versus 79% in the IV-treatment group.5Four patients in the parenteral group required dosage modification because of serious AEs. Findings suggest equivalent therapeutic outcomes for oral ciprofloxacin and parenteral therapy.
In a comparative trial, 14 patients with osteomyelitis received oral ciprofloxacin 750 mg twice daily for 6 weeks or longer.11 The remaining 16 patients received other antimicrobial agents. At 13-month follow-up, 50% (7/14) of ciprofloxacin patients were cured of infection.11 The cure rate for patients with Pseudomonas infections was 37% (3/8). Eleven patients taking other antimicrobials were cured of infection and experienced wound healing during follow-up of 1 to 13 months.11
In another study, sequential administration of parenteral and oral ciprofloxacin was compared with IV ceftazidime in osteomyelitis patients. Patients received either IV ciprofloxacin 200 mg for 12 hours followed by 500 mg twice daily by mouth or IV ceftazidime 2 g for 12 hours.12Two of three patients were cured after sequential administration of ciprofloxacin, as were all three patients receiving ceftazidime.12
In a prospective, randomized, open-label trial, patients with chronic osteomyelitis received oral ofloxacin 400 mg twice daily (n = 16) or IV imipenem-cilastatin 500 mg every 6 hours (n = 16).13 (Imipenem is a beta-lactam antibiotic; cilastatin is an enzyme inhibitor that prevents degradation of imipenem.) Cure rates for the ofloxacin and imipenem groups were 69% and 50%, respectively.13
In another trial, oral ofloxacin was compared with parenteral agents for treating chronic osteomyelitis.14 Nineteen patients without prosthetic implants received oral ofloxacin 400 mg twice daily for a mean of 8 weeks; another 14 patients received parenteral cefazolin (1 g/8 h IV) or ceftazidime (2 g/12 h) for 4 weeks.14 Surgical debridement was performed prior to the trial. For P aeruginosa infections, ceftazidime was administered every 8 hours. A complete ofloxacin regimen was considered to be 6 weeks; for parenteral treatment, a complete course was 4 weeks. Therapy was continued beyond a course if the patient’s condition showed some improvement. Long-term assessment suggested that 74% (14/19) of ofloxacin patients and 86% (12/14) of parenteral patients were cured of infection with no relapse.14 Importantly, three patients with DM had complete resolution of infection. There was no statistical difference between cure rates. Three ofloxacin patients and one parenteral patient had relapse of infection.14 Drug-related AEs were nausea, insomnia, rash, and ophthalmic reaction.14
In an open-label trial, the efficacy and safety of ciprofloxacin versus levofloxacin were studied in patients with chronic osteomyelitis.15 Oral ciprofloxacin was dosed at 750 mg twice daily; oral levofloxacin was dosed at 500 mg daily for an average of 60 days. Patients were monitored for up to 36 months. Nine of 15 levofloxacin patients were cured (60%); the cure rate in the ciprofloxacin-treated group was 40%.15 Infection resolved in 11 of the 18 patients suffering from staphylococcal infections following completion of the antibiotic course.15
Rifampin: In one study, patients with staphylococcal infections from prosthetic devices were treated with rifampin plus ciprofloxacin or ciprofloxacin plus placebo for 3 to 6 months.16 Eighteen patients were randomly assigned to ciprofloxacin plus rifampin; another 15 received ciprofloxacin plus placebo.16 Oral ciprofloxacin was dosed at 750 mg every 12 hours, and oral rifampin at 450 mg every 12 hours. Twenty-four patients completed the trial. In the ciprofloxacin-rifampin group, the cure rate was 100% (12/12), whereas 58% of ciprofloxacin-placebo patients achieved resolution of signs and symptoms.16 Seven dropouts were later treated with rifampin combinations, with three patients on a reduced rifampin regimen (300 mg/12 h). Rifampin combinations resulted in a 71% cure in initial dropouts.16
Therapeutic effects of rifampin added to parenteral oxacillin (penicillinase-resistant penicillin) or vancomycin (bacterial cell wall synthesis inhibitor) were examined in patients with S aureus bone infections.17 Rifampin-combination patients (n = 10) received IV oxacillin 3 g every 6 hours or IV vancomycin 1 g in 12 hours plus oral rifampin (600 mg/day) for approximately 21 days. The placebo group (n = 13) received oxacillin or vancomycin plus oral phenazopyridine 150 mg twice daily. Ninety percent of patients were cured when rifampin was added to the parenteral regimen, versus 62% cured in the placebo group.17
Similarly, rifampin was evaluated in combination with a penicillinase-resistant penicillin (nafcillin). Ten patients with chronic osteomyelitis received IV nafcillin (20 mg/kg/4 h) and oral rifampin (600 mg twice daily) for a mean of 36 weeks.18 In another treatment group, four patients received parenteral nafcillin only. In follow-up of 2 to 4 years, eight patients in the combined-treatment group were cured (80%), whereas the cure rate was 50% in the nafcillin-only group.18 The only AE reported was mild neutropenia in four patients.
Linezolid: Oral or parenteral linezolid was compared with IV ampicillin-sulbactam or oral amoxicillin-clavulanate in a large, randomized, open-label, multicenter diabetic foot infection trial that included a subset of patients with chronic osteomyelitis.19 Patients received linezolid (600 mg/12 h by mouth or IV), ampicillin-sulbactam (1.5-3 g/6 h IV), or amoxicillin-clavulanate (500-875 mg/8-12 h by mouth).19 Treatment duration was 7 to 28 days. Vancomycin (1 g/12 h) could be added to the aminopenicillin and beta-lactamase inhibitor if MRSA infection was suspected or confirmed. Fifty-seven osteomyelitis patients received linezolid and 20 received an aminopenicillin and beta-lactamase inhibitor combination. Cure rates were 61% and 69% for linezolid patients and aminopenicillin-plus-beta-lactamase inhibitor patients, respectively.19 Drug-related AEs reported in linezolid patients were nausea, anemia, and thrombocytopenia.19
SMX-TMP: Fifty patients with chronic S aureus osteomyelitis who underwent surgical debridement were recruited in a randomized, comparative trial.20 Group A patients (n = 22) received IV cloxacillin (a penicillinase-resistant penicillin) 2 g every 4 hours for 6 weeks, followed by oral cloxacillin 500 mg every 6 hours for another 2 weeks. Group B patients (n = 28) received a rifampin-cotrimoxazole combination (800 mg oral rifampin/day and oral TMP 7-8 mg/kg/day) for 8 weeks.20 The TMP dosage was three 80/400 mg cotrimoxazole tablets every 24 hours. The overall cure rate in both groups at 10-year follow-up was approximately 90%, suggesting that oral SMX-TMP was an effective treatment option for the condition.20
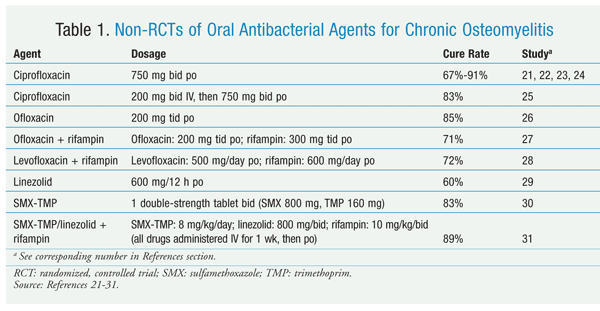
In addition to these clinical studies, oral antibacterial drugs were evaluated for chronic osteomyelitis in several nonrandomized clinical trials (TABLE 1).21-31
Conclusion
Oral antibacterial agents may serve as effective alternatives to parenteral therapy for the management of chronic osteomyelitis. At the same time, it is important to note that the number of randomized, clinical trials evaluating the efficacy and safety of oral antibiotics is far from adequate. Treatment regimens are currently selected based upon case reports, personal experience, and preclinical data. To improve this situation, multicenter comparative trials including sufficient numbers of patients are warranted. Findings from in-depth studies can pave the way to developing a treatment algorithm for this critical disease state.
REFERENCES
1. Hatzenbuehler J, Pulling TJ. Diagnosis and management of osteomyelitis. Am Fam Physician. 2011;84:1027-1033.
2. Boucher H, Miller LG, Razonable RR. Serious infections caused by methicillin-resistant Staphylococcus aureus. Clin Infect Dis.2010;51(suppl 2): S183-S197.
3. Walter G, Kemmerer M, Kappler C, Hoffmann R. Treatment algorithms for chronic osteomyelitis. Dtsch Arztebl Int. 2012;109:257-264.
4. Tice AD, Hoaglund PA, Shoultz DA. Outcomes of osteomyelitis among patients treated with outpatient parenteral antimicrobial therapy. Am J Med. 2003;114:723-728.
5. Gentry LO, Rodriguez GG. Oral ciprofloxacin compared with parenteral antibiotics in the treatment of osteomyelitis. Antimicrob Agents Chemother. 1990;34:40-43.
6. Cipro (ciprofloxacin) product information. Wayne, NJ: Bayer Healthcare Pharmaceuticals Inc; September 2008.
7. Rimactane (rifampin) product information. Broomfield, CO: Sandoz Inc; 1971.
8. Zyvox (linezolid) product information. New York, NY: Pharmacia and Upjohn Co; March 2007.
9. Bactrim (sulfamethoxazole and trimethoprim) product information. Philadelphia, PA: Mutual Pharmaceutical Co, Inc; March 2005.
10. Chambers HF, Deck DH. Beta-lactam & other cell wall- & membrane-active antibiotics. In: Katzung BG, Masters SB, Trevor AJ.Basic and Clinical Pharmacology. 11th ed. New York, NY: McGraw Hill Medical; 2009:773-793.
11. Greenberg RN, Tice AD, Marsh PK, et al. Randomized trial of ciprofloxacin compared with other antimicrobial therapy in the treatment of osteomyelitis. Am J Med. 1987;82:266-269.
12. Peacock JE Jr, Pegram PS, Weber SF, Leone PA. Prospective, randomized comparison of sequential intravenous followed by oral ciprofloxacin with intravenous ceftazidime in the treatment of serious infections. Am J Med. 1989;87: 185S-190S.
13. Gomis M, Barberán J, Sánchez B, et al. Oral ofloxacin versus parenteral imipenem-cilastatin in the treatment of osteomyelitis. Rev Esp Quimioter. 1999;12:244-249.
14. Gentry LO, Rodriguez-Gomez G. Ofloxacin versus parenteral therapy for chronic osteomyelitis. Antimicrob Agents Chemother.1991;35:538-541.
15. Greenberg RN, Newman MT, Shariaty S, Pectol RW. Ciprofloxacin, lomefloxacin, or levofloxacin as treatment for chronic osteomyelitis. Antimicrob Agents Chemother. 2000;44:164-166.
16. Zimmerli W, Widmer AF, Blatter M, et al. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. JAMA. 1998;279:1537-1541.
17. Van der Auwera P, Klastersky J, Thys JP, et al. Double-blind, placebo-controlled study of oxacillin combined with rifampin in the treatment of staphylococcal infections. Antimicrob Agents Chemother. 1985;28:467-472.
18. Norden CW, Bryant R, Palmer D, et al. Chronic osteomyelitis caused by Staphylococcus aureus: controlled clinical trial of nafcillin therapy and nafcillin-rifampin therapy. South Med J. 1986;79:947-951.
19. Lipsky BA, Itani K, Norden C. Treating foot infections in diabetic patients: a randomized, multicenter, open-label trial of linezolid versus ampicillin-sulbactam/amoxicillin-clavulanate. Clin Infect Dis. 2004;38:17-24.
20. Euba G, Murillo O, Fernández-Sabé N, et al. Long-term follow-up trial of oral rifampin-cotrimoxazole combination versus intravenous cloxacillin in treatment of chronic staphylococcal osteomyelitis. Antimicrob Agents Chemother. 2009;53:2672-2676.
21. Lesse AJ, Freer C, Salata RA, et al. Oral ciprofloxacin therapy for gram-negative bacillary osteomyelitis. Am J Med. 1987;82:247-253.
22. Hessen MT, Ingerman MJ, Kaufman DH, et al. Clinical efficacy of ciprofloxacin therapy for gram-negative bacillary osteomyelitis. Am J Med. 1987;82:262-265.
23. Mader JT, Cantrell JS, Calhoun J. Oral ciprofloxacin compared with standard parenteral antibiotic therapy for chronic osteomyelitis in adults. J Bone Joint Surg Am. 1990;72:104-110.
24. Giamarellou H, Galanakis N. Use of intravenous ciprofloxacin in difficult-to-treat infections. Am J Med. 1987;82:346-351.
25. Scully BE, Neu HC. Treatment of serious infections with intravenous ciprofloxacin. Am J Med. 1987;82:369-375.
26. Ketterl R, Beckurts T, Stübinger B, Claudi B. Use of ofloxacin in open fractures and in the treatment of post-traumatic osteomyelitis. J Antimicrob Chemother. 1988;22(suppl C):159-166.
27. Drancourt M, Stein A, Argenson JN, et al. Oral rifampin plus ofloxacin for treatment of Staphylococcus-infected orthopedic implants. Antimicrob Agents Chemother. 1993;37:1214-1218.
28. Barberán J, Aguilar L, Giménez MJ, et al. Levofloxacin plus rifampicin conservative treatment of 25 early staphylococcal infections of osteosynthetic devices for rigid internal fixation. Int J Antimicrob Agents. 2008;32:154-157.
29. Birmingham MC, Rayner CR, Meagher AK, et al. Linezolid for the treatment of multidrug-resistant, gram-positive infections: experience from a compassionate-use program. Clin Infect Dis. 2003;36:159-168.
30. Craven JL, Pugsley DJ, Blowers R. Trimethoprim-sulphamethoxazole in acute osteomyelitis due to penicillin-resistant staphylococci in Uganda. Br Med J. 1970;3:201-203.
31. Nguyen S, Pasquet A, Legout L, et al. Efficacy and tolerance of rifampicin-linezolid compared with rifampicin-cotrimoxazole combinations in prolonged oral therapy for bone and joint infections. Clin Microbiol Infect. 2009;15:1163-1169.





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 