N Engl J Med 2014; 370:970-971March 6, 2014DOI: 10.1056/NEJMcibr1315706
Cancer cells use diverse means to avoid being damaged by drugs. Often, the development of new treatment strategies in clinical oncology is made possible by discovering the mechanisms of drug resistance by tumors. However, the development of treatments that target such mechanisms is particularly challenging when an intact mechanism is required for a human to sustain life. A recent study by Arora and colleagues1 suggests that a shift may occur in advanced prostate cancer — from the expression of a steroid receptor that is not required for life to another that is — and that this shift allows the cancer to sustain tumor signaling essential for disease progression.
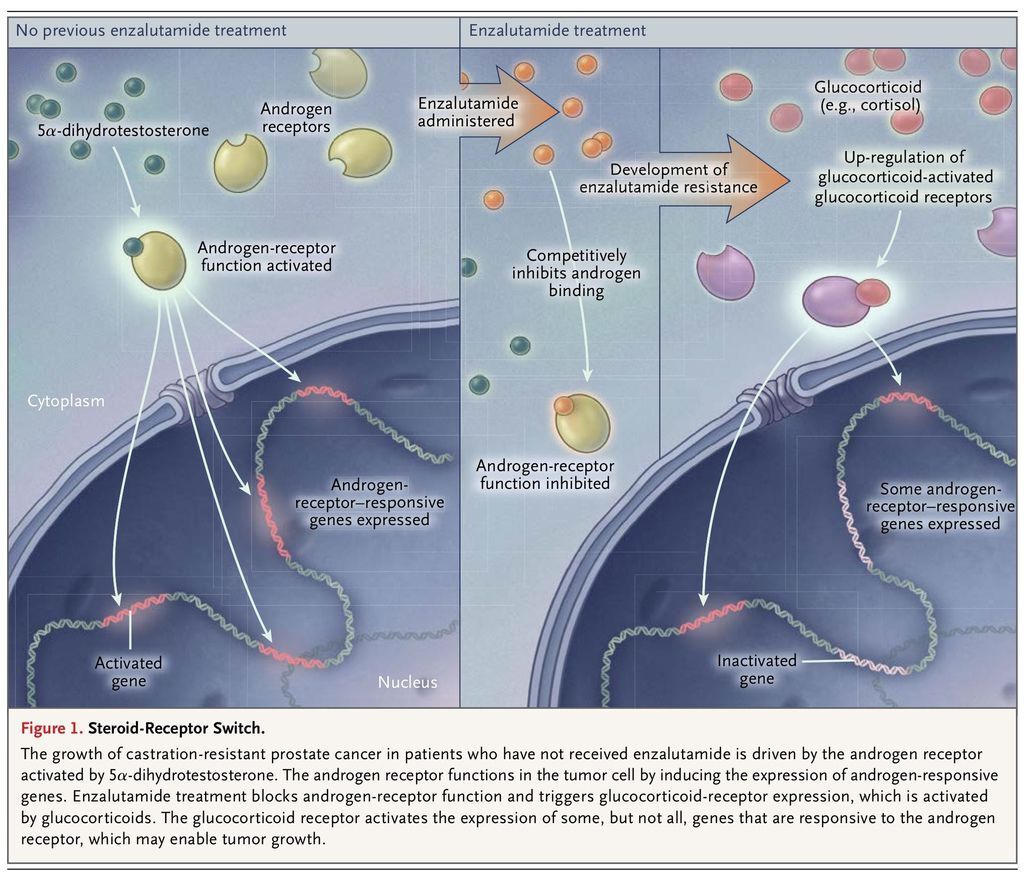
For decades, the standard treatment for metastatic prostate cancer has been androgen-deprivation therapy by means of medical or surgical castration. Despite a high rate of initial response, tumors eventually progress as castration-resistant prostate cancer. Sustained androgen-receptor signaling is a major driver of castration-resistant prostate cancer, owing to the contributions from the increased expression of the androgen receptor2 and an acquired capability of tumors to more rapidly convert precursor steroids to 5α-dihydrotestosterone, the most potent androgen, as compared with untreated tumors.3 The requirement for sustained androgen-receptor signaling in castration-resistant prostate cancer has been clinically validated by phase 3 studies that have shown an increase in survival among patients treated with the androgen-synthesis inhibitor abiraterone and the potent androgen-receptor antagonist enzalutamide.4
However, even the responses to this new generation of hormonal therapies targeting the androgen receptor are temporary, and the tumor becomes refractory to their effects. Now, investigators in the field are asking how tumors become resistant to abiraterone and enzalutamide and whether the androgen receptor still might play a role in this context. Arora and colleagues found that exposure of a preclinical model of prostate cancer to enzalutamide led to the induction of glucocorticoid-receptor expression in a subset of cells within just a few days, which then evolved to massive glucocorticoid-receptor up-regulation with the development of enzalutamide resistance (Figure 1
FIGURE 1
Steroid-Receptor Switch.
). They observed that glucocorticoid-receptor expression in disseminated tumor cells from patients with castration-resistant prostate cancer was associated with a poor clinical response to enzalutamide.
Moreover, in models of prostate cancer, dexamethasone spurs the development of enzalutamide resistance, whereas treatment with a pharmacologic glucocorticoid-receptor antagonist, or genetically silencing glucocorticoid-receptor expression, restores enzalutamide sensitivity. In this context, the activation of the glucocorticoid receptor induces the expression of an overlapping, although not identical, set of genes, as compared with the activation of the androgen receptor. In the face of a tighter squeeze on the androgen receptor (in the form of second-line and third-line hormonal therapies) in castration-resistant prostate cancer, it appears that an alternative steroid receptor assumes the usual role of the androgen receptor.
The androgen receptor is not essential for life, as shown by the viability of patients who have complete androgen insensitivity syndrome.5 A negative effect on essential physiological functions has therefore not been a barrier generally to the development of intensified inhibition of the androgen receptor in the treatment of advanced prostate cancer. In contrast, the glucocorticoid receptor is indispensable for life. This situation presents an inherent challenge in clinically testing the requirement for glucocorticoid-receptor signaling in enzalutamide-resistant prostate cancer with the use of pharmacologic interventions.
Yet another challenge is whether and how to integrate these data with current clinical practice. Glucocorticoids have long played an important role in the treatment of prostate cancer, to the point that glucocorticoids are commonly used in the comparator group of phase 3 trials. These agents are often used for palliation and probably work in part through the suppression of adrenal androgens. If tumor expression of the glucocorticoid receptor spurs clinical resistance to enzalutamide, is its relevance exclusive to the context of enzalutamide therapy? Is it possible that glucocorticoid administration has direct effects on the tumor that may be detrimental to the patient in other clinical settings? The answers to these questions will depend in part on the collection of tumor tissue from patients with castration-resistant prostate cancer, which will permit the further investigation of molecular features involved in resistance to systemic therapies.





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 