HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use HUMIRA® safely and effectively. See full prescribing information for HUMIRA.
HUMIRA (adalimumab) solution for subcutaneous injection
Initial U.S. Approval: 2002
RECENT MAJOR CHANGES —————––––––––––––––––––––––––––
Indications and Usage, Psoriatic Arthritis (1.2) 11/2006
Indications and Usage, Ankylosing Spondylitis (1.3) 7/2006
Indications and Usage, Crohn’s Disease (1.4) 2/2007
Dosage and Administration, Ankylosing Spondylitis (2.1) 7/2006
Dosage and Administration, Crohn’s Disease (2.2) 2/2007
Warnings and Precautions, Serious Infections (5.1) 2/2007
Warnings and Precautions, Malignancies (5.2) 2/2007
Warnings and Precautions, Hepatitis B Virus Reactivation (5.4) 6/2006
Warnings and Precautions, Immunizations (5.10) 2/2007
WARNING: RISK OF SERIOUS INFECTIONS
See full prescribing information for complete boxed warning.
Tuberculosis (TB), invasive fungal, and other opportunistic infections, some fatal, have occurred. Perform test for latent
TB; if positive, start treatment for TB prior to starting HUMIRA. Monitor all patients for active TB during treatment,even if initial latent TB test is negative (5.1)
INDICATIONS AND USAGE —————–––––––––––––––––––––––––––
HUMIRA is a tumor necrosis factor (TNF) blocker indicated for treatment of:
Rheumatoid Arthritis (RA) (1.1)(類風濕性關節炎)
• Reducing signs and symptoms, inducing major clinical response, inhibiting the progression of structural damage, and improving physical function in adult patients with moderately to severely active disease.
Psoriatic Arthritis (1.2)(牛皮癬性關節炎)
• Reducing signs and symptoms of active arthritis, inhibiting the progression of structural damage, and improving physical function.
Ankylosing Spondylitis (1.3)(僵直性脊椎炎)
• Reducing signs and symptoms in patients with active disease.
Crohn’s Disease (1.4)
• Reducing signs and symptoms and inducing and maintaining clinical remission in adult patients with moderately to severely active Crohn’s disease who have had an inadequate response to conventional therapy. Reducing signs and symptoms and inducing clinical remission in these patients if they have also lost response to or are intolerant to infliximab.
DOSAGE AND ADMINISTRATION —————–––––––––––––––––––––––
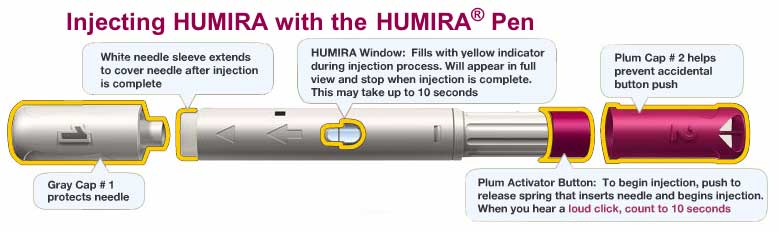
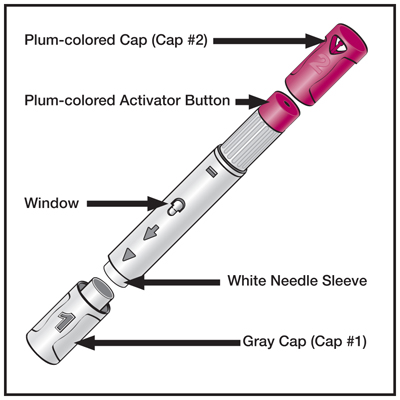
Humira is administered by subcutaneous injection.
Rheumatoid Arthritis, Psoriatic Arthritis, Ankylosing Spondylitis (2.1)
• 40 mg every other week. Some patients with RA not receiving methotrexate may benefit from increasing the frequency to
40 mg every week.
Crohn’s Disease (2.2)
• 160 mg initially at Week 0, 80 mg at Week 2, followed by a maintenance dose of 40 mg every other week beginning at Week
4. Initial dose may be given as 4 injections on 1 day, or divided over 2 days.
DOSAGE FORMS AND STRENGTHS ————–––––––––––––––––––––––
• 40 mg/0.8 mL in a single-use prefilled pen (HUMIRA Pen) (3)
• 40 mg/0.8 mL in a single-use prefilled glass syringe (3)
CONTRAINDICATIONS —————––––––––––––––––––––––––––––
• None (4)
WARNINGS AND PRECAUTIONS ————–––––––––––––––––––––––––
• Serious infections – do not start HUMIRA during an active infection. If an infection develops, monitor carefully, and stop
HUMIRA if infection becomes serious (5.1)
• Malignancies – are seen more often than in controls, and lymphoma is seen more often than in the general population (5.2)
• Anaphylaxis or serious allergic reactions may occur (5.3)
• Hepatitis B virus reactivation – monitor HBV carriers during and several months after therapy. If reactivation occurs, stop
HUMIRA and begin anti-viral therapy (5.4)
• Demyelinating disease, exacerbation or new onset, may occur (5.5)
• Cytopenias, pancytopenia – advise patients to seek immediate medical attention if symptoms develop, and consider stopping
HUMIRA (5.6)
• Heart failure, worsening or new onset, may occur (5.8)
• Lupus-like syndrome – stop HUMIRA if syndrome develops (5.9)
ADVERSE REACTIONS —————––––––––––––––––––––––––––––
Most common adverse reactions (incidence >10%): infections (e.g. upper respiratory, sinusitis), injection site reactions,
headache and rash (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Abbott Laboratories at 1-800-633-9110 or FDA at
1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS ——––––———––––––––––––––––––––––––
• Anakinra – increased risk of serious infection (5.7, 7.1)
• Live vaccines – should not be given with HUMIRA (5.10, 7.2)
USE IN SPECIFIC POPULATIONS ————––—–––––––––––––––––––––
• Pregnancy: Physicians are encouraged to enroll pregnant patients in the HUMIRA pregnancy registry by calling 1-877-311-8972 (8.1)


資料來源:www.humira.com/





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 