大便微生物膠囊用來治療Clostridium difficile感染,或許用糞便微生物移植這個說法會比較容易接受,當然不是真的叫你吃大便啦!又不是中藥的五靈脂。
而是健康人體排出的糞便裡面的微生物分佈跟腸道裡的情形應該差不多,因此如果把他們移植到病患體內,就可以用健康的腸道菌種分佈來改善病人的腸道功能,用這種方式來治療「困難梭狀桿菌」(Clostridium difficile)
ABSTRACT
Importance Fecal microbiota transplantation (FMT) has been shown to be effective in treating relapsing or refractory Clostridium difficile infection, but practical barriers and safety concerns have prevented its widespread use.
Objective
To evaluate the safety and rate of resolution of diarrhea following administration of frozen FMT capsules from prescreened unrelated donors to patients with recurrent C difficile infection.
Design, Setting, and Participants Open-label, single-group, preliminary feasibility study conducted from August 2013 through June 2014 at Massachusetts General Hospital, Boston. Twenty patients (median age, 64.5 years; range, 11-89 years) with at least 3 episodes of mild to moderate C difficile infection and failure of a 6- to 8-week taper with vancomycin or at least 2 episodes of severe C difficile infection requiring hospitalization were enrolled.
Interventions Healthy volunteers were screened as potential donors and FMT capsules were generated and stored at −80°C (−112°F). Patients received 15 capsules on 2 consecutive days and were followed up for symptom resolution and adverse events for up to 6 months.
Main Outcomes and Measures The primary end points were safety, assessed by adverse events of grade 2 or above, and clinical resolution of diarrhea with no relapse at 8 weeks. Secondary end points included improvement in subjective well-being per standardized questionnaires and daily number of bowel movements.
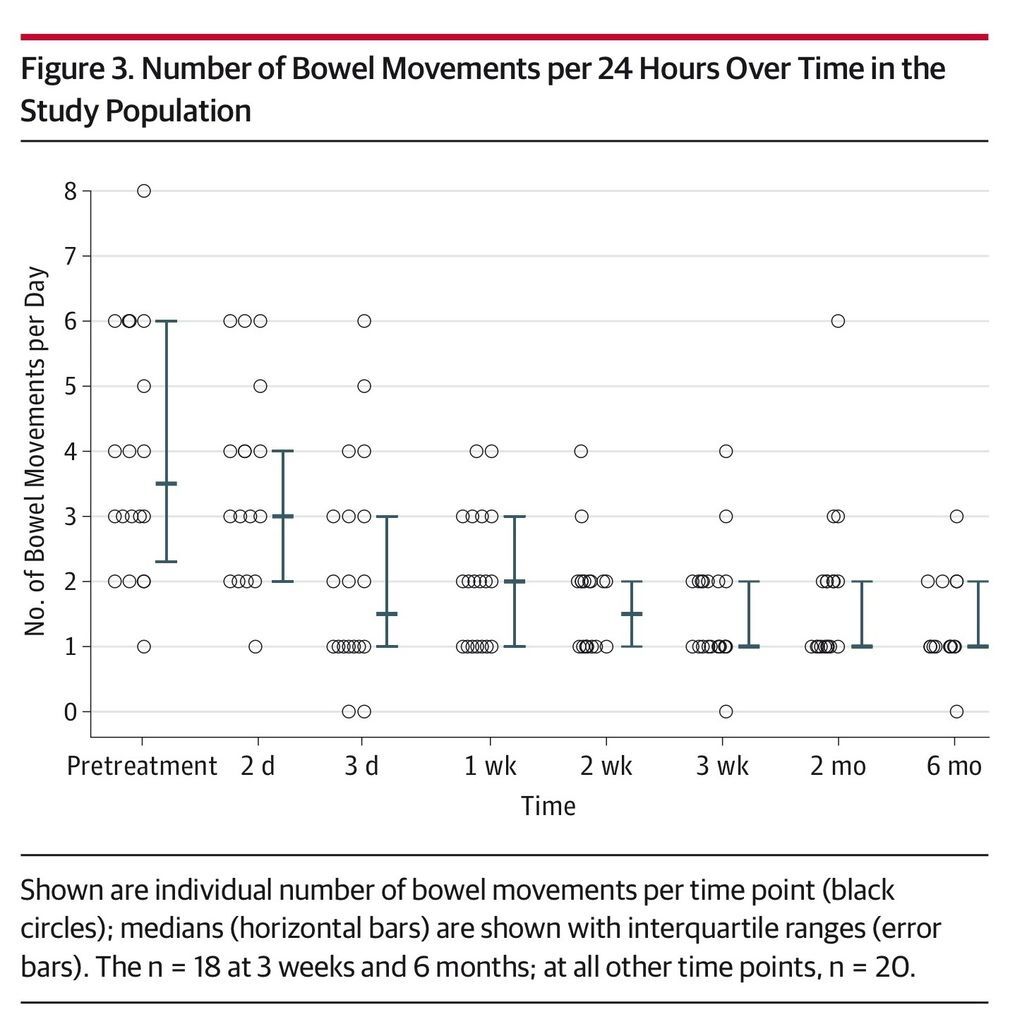
Results No serious adverse events attributed to FMT were observed. Resolution of diarrhea was achieved in 14 patients (70%; 95% CI, 47%-85%) after a single capsule-based FMT. All 6 nonresponders were re-treated; 4 had resolution of diarrhea, resulting in an overall 90% (95% CI, 68%-98%) rate of clinical resolution of diarrhea (18/20). Daily number of bowel movements decreased from a median of 5 (interquartile range [IQR], 3-6) the day prior to administration to 2 (IQR, 1-3) at day 3 (P = .001) and 1 (IQR, 1-2) at 8 weeks (P < .001). Self-ranked health scores improved significantly on a scale of 1 to 10 from a median of 5 (IQR, 5-7) for overall health and 4.5 (IQR, 3-7) for gastrointestinal-specific health on the day prior to FMT to 8 (IQR, 7-9) after FMT administration for both overall and gastrointestinal health (P = .001). Patients needing a second treatment to obtain resolution of diarrhea had lower pretreatment health scores (median, 6.5 [IQR, 5-7.3] vs 5 [IQR, 2.8-5]; P = .02).
Conclusions and Relevance This preliminary study among patients with relapsing C difficileinfection provides data on adverse events and rates of resolution of diarrhea following administration of FMT using frozen encapsulated inoculum from unrelated donors. Larger studies are needed to confirm these results and to evaluate long-term safety and effectiveness.
Trial Registration clinicaltrials.gov Identifier: NCT01914731
Recurrent and refractory Clostridium difficile infection (CDI) is a major cause of morbidity and mortality, with a recent increase in the number of adult and pediatric patients affected globally.1- 4 Standard treatment with oral administration of metronidazole or vancomycin is increasingly associated with treatment failures, and disease recurrence has been described in up to 30% of patients after their first episode and up to 60% after 2 or more recurrences.5- 7 The emergence of a virulent strain of the organism (NAP1/BI/027) has been associated with even higher rates of treatment failure.8 Fidaxomicin has been shown to reduce the rate of recurrence compared with vancomycin; however, its use has not been studied in patients with multiple recurrences and is limited by its cost.9,10
Fecal microbiota transplantation (FMT)—ie, reconstitution of normal flora by a stool transplant from a healthy individual—has been shown to be effective in treating relapsing CDI.11- 14 The majority of reported FMT procedures have been performed with fresh stool suspensions from related donors. This approach has practical barriers that hinder the development of scientifically sound treatment protocols. The use of fresh donations requires prior identification and screening of a suitable donor, thus precluding the use of FMT in acute situations. Furthermore, the limited viability of fresh samples, usually estimated at up to 6 hours, makes thorough screening of donors and donation aliquots impractical.
In an attempt to address these concerns, we recently described the successful use of frozen FMT inocula from carefully screened healthy volunteer donors for treating CDI and showed that nasogastric tube administration of a frozen inoculum was comparable with colonoscopic delivery.15 Building on this work, we generated a capsulized frozen inoculum that can be administered orally and obviates the need for any gastrointestinal procedures. The aim of this study was to evaluate the safety and rate of diarrhea resolution associated with use of this treatment for patients with CDI.
An open-label, single-group preliminary feasibility study was undertaken to evaluate the safety and rate of diarrhea resolution following administration of frozen FMT capsules for treatment of a small cohort of patients with relapsing or recurrent CDI. The study was approved by the Partners Human Research Committee. The investigation was determined to be exempt from review by the US Food and Drug Administration per guidance recently published by the Center for Biologics Evaluation and Research.16,17All adult participants provided written informed consent after a clinical meeting with a physician investigator providing information about potential risks and benefits of the procedure. Children aged 7 years or older provided assent in addition to parental informed consent.
Study Population and Settings
The study was conducted at Massachusetts General Hospital, Boston. Participants were recruited by referrals from colleagues at Partners Healthcare, of which Massachusetts General Hospital is a member. Patients aged 7 to 90 years with refractory or recurrent CDI were included, as defined in consensus guidelines18 as a relapse of CDI after having at least 3 episodes of mild to moderate CDI and failure of a 6- to 8-week taper with vancomycin with or without an alternative antibiotic or at least 2 episodes of severe CDI resulting in hospitalization and associated with significant morbidity. Active CDI was defined as diarrhea (>3 loose stools per day) with a positive stool test result. Clostridium difficile testing in our institution is by initial toxin and glutamate dehydrogenase enzyme-linked immunosorbent assays, followed by polymerase chain reaction if the toxin test result is negative but the glutamate dehydrogenase test result is positive or indeterminate. Exclusion criteria included delayed gastric emptying syndrome, recurrent aspirations, pregnancy, significantly compromised immunity, and having a history of significant allergy to foods not excluded from the donor diet. A full list of inclusion/exclusion criteria can be found in eAppendix 1 in the Supplement.
Donor Screening
Donors were healthy, nonpregnant adults aged 18 to 50 years, taking no medications, and with a normal body mass index (18.5-25 [calculated as weight in kilograms divided by height in meters squared]). Volunteers were excluded for any significant medical history (with the exception of resolved traumatic injury) or for any use of antibiotics in the preceding 6 months. Candidates passed the American Association of Blood Banks donor questionnaire,19 then underwent physical examination and general laboratory screening tests. Donor feces were screened for enteric pathogens and blood was screened for antibodies to hepatitis A, B, and C; human immunodeficiency virus; and Treponema pallidum within 2 weeks of donations. The volunteers were asked to refrain from eating common allergens within 5 days of stool donation but otherwise not to alter their diets. At the time of donation, they had an interim health query for febrile, systemic, and gastrointestinal symptoms and were deferred for any change in health status. All donations were stored without use for an additional 4 weeks to allow retesting of donors for human immunodeficiency virus and hepatitis B and C prior to clinical use of the inoculum (full donor exclusion criteria and screening protocol can be found in eAppendixes 2 and 3 in the Supplement). Donors were recruited by public advertising and paid a small stipend.
Preparation of Frozen Inocula
Processing was carried out under aerobic conditions. A fecal suspension was generated in normal saline without preservatives using a commercial blender. Materials were sequentially sieved to remove particulate material. The final slurry was concentrated by centrifugation and resuspended in saline at one-tenth the volume of the initial sample with 10% glycerol added as a bacterial cryoprotectant. Fecal matter solution was pipetted into size 0 capsules (650 µL), which were closed and then secondarily sealed in size 00 capsules. Capsules were stored frozen at −80°C (−112°F). One to two hours prior to administration, they were transferred to −20°C, then transported to the clinic on dry ice. Commercially available acid-resistant hypromellose capsules (DRCaps, Capsugel) were used. Stability of capsules in an acid environment mimicking the stomach was tested internally by evaluating trypan blue–filled capsules. At 37°C (99°F) and a pH of 3 or less, the capsules were stable for 115 minutes before dye was released. These results are comparable with data published after we conducted our internal evaluation.20 Each inoculum was prepared from the feces of a single donor and a full treatment of 30 capsules contained sieved, concentrated material derived from a mean of 48 g of fecal matter (mean per capsule, 1.6 g; range, 1.0-2.05 g).
Study Procedures
Patients were required to discontinue antibiotics for CDI 48 hours prior to FMT and asked to fast for 4 hours prior to and 1 hour following capsule intake. Participants were given 15 capsules, handed individually to the patient by an investigator, on 2 consecutive days. Patients who showed no improvement in diarrheal symptoms after 72 hours were retested and offered re-treatment for positive results. To minimize potential infectious exposures, inoculum from the same donor was used for the repeat administration. Patients with symptomatic improvement were not retested for C difficile, as recommended in practice guidelines.21,22Participants were followed up with structured questionnaires administered on days 1, 2, 3, 7, 14, and 21 and 2 and 6 months after the procedure (eAppendix 4 in the Supplement). Questionnaires recorded stool frequency and consistency, general and gastrointestinal well-being via a standardized health score, rating of gastrointestinal symptoms, medication use, and weight changes. Questions related to the previous 24 hours. Overall and gastrointestinal-specific health scores were reported on a scale of 1 to 10, with 1 being the lowest and 10 being “best possible health for you.” Possible adverse events were elicited by use of a modification of the Common Terminology Criteria for Adverse Events version 3.0.23 Fever, gastrointestinal symptoms, headache, fatigue, and rash were the main symptoms evaluated (eAppendix 5 in the Supplement).
Outcomes
The primary end points were safety, defined by any FMT-related adverse events at grade 2 or above, and clinical resolution of diarrhea while not receiving antibiotics for C difficile without relapse within 8 weeks. For patients who required a second treatment, follow-up was calculated starting at the time of the second administration. Resolution of diarrhea was defined as fewer than 3 bowel movements per 24 hours. Secondary end points included improvement in subjective well-being per standardized questionnaire and daily number of bowel movements.
Data Analysis
The sample size was calculated using the prior published rate of diarrhea resolution of 30% with standard antimicrobial treatment among patients with more than 3 relapses of CDI.24 Assuming a rate of clinical resolution of diarrhea of 90% among study participants, we estimated the target sample size at 19 patients (2-tailed α = .05; 1 − β = .90).
Continuous variables were tested for normal distribution using the Kolmogorov-Smirnov test. Categorical variables were compared between patients who achieved resolution of diarrhea and those in whom the first treatment failed using the χ2 test or Fisher exact test and continuous variables using the Mann-Whitney test. In addition, a logistic regression model with mixed effects was used to identify factors associated with diarrhea resolution after 1 treatment while controlling for clustering within donors. Wilcoxon signed rank, Friedman, and McNemar tests were used to compare variables before and after treatment. A linear mixed model was used to assess for variables potentially related to the secondary outcomes (bowel movements per day, gastrointestinal health score, and overall health score) while controlling for clustering within donors. Bowel movements per day were compared with normal using a 1-sample Wilcoxon signed rank test. The Fisher exact test was used to compare rates of successful treatment with our previous study and an exact binomial test to compare with historically reported rates of successful treatment. As we had few missing data throughout follow-up (4 of 140 data points [2.8%]), no imputation was performed. A 2-tailed P < .05 was considered statistically significant. Analyses were performed with R version 3.1.1 and SPSS version 22 (SPSS Inc) software.
From July 2013 through January 2014, a total of 20 recipients were treated using stools obtained from 4 donors. All consecutive patients meeting study inclusion criteria were offered enrollment. Six-month follow-up was completed in July 2014. Baseline recipient characteristics are shown in Table 1. Capsules were stored in −80°C (−112°F) for a mean of 113 days (range, 30-252 days) prior to administration.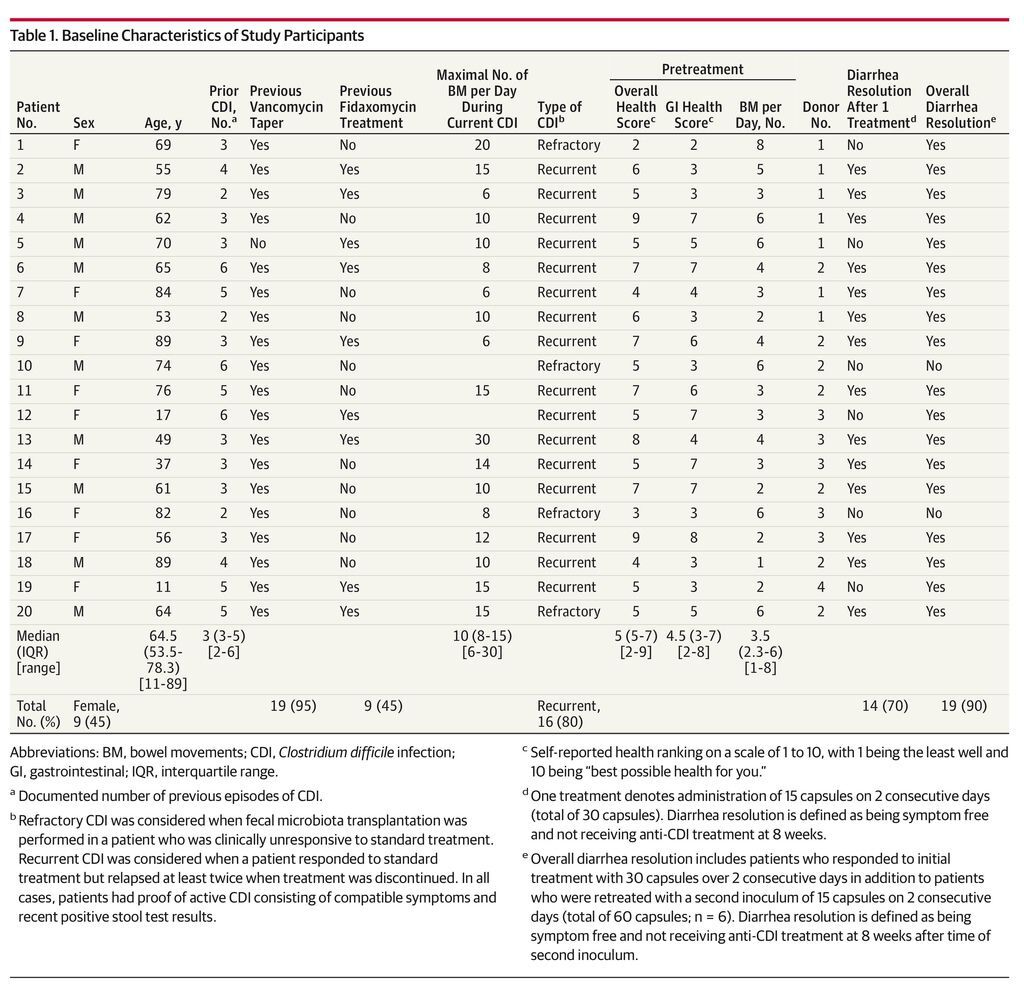
Primary Outcome
No serious adverse events (grade 2 or above) were observed. Among 20 patients treated, 14 had clinical resolution of diarrhea after the first administration of FMT (70%; 95% CI, 47%-85%) and remained symptom free at 8 weeks. All 6 nonresponders were re-treated at a mean of 7 days (SD, 2.1 days) after the first procedure. Of these 6, 5 patients had resolution of diarrhea after the second treatment (19/20 [95%]). However, 1 patient relapsed within the predetermined 8-week follow-up after initial diarrhea resolution, resulting in an overall rate of diarrhea resolution of 90% (95% CI, 68%-98%). The only variable significantly associated with response to first treatment was overall health score prior to FMT (Table 2). Patients who needed a second treatment to achieve resolution of diarrhea had lower pretreatment health scores (were more symptomatic) than patients who had diarrhea resolution after a single administration (median health scores, 6.5 [interquartile range {IQR}, 5-7.3] vs 5 [IQR, 2.8-5], respectively; P = .02). Using a logistic regression model controlling for donor clustering, lower pretreatment health scores were associated with resolution of diarrhea after 1 treatment (odds ratio, 3.18; 95% CI, 0.99-10.31; P = .05) (eTable 1 in the Supplement).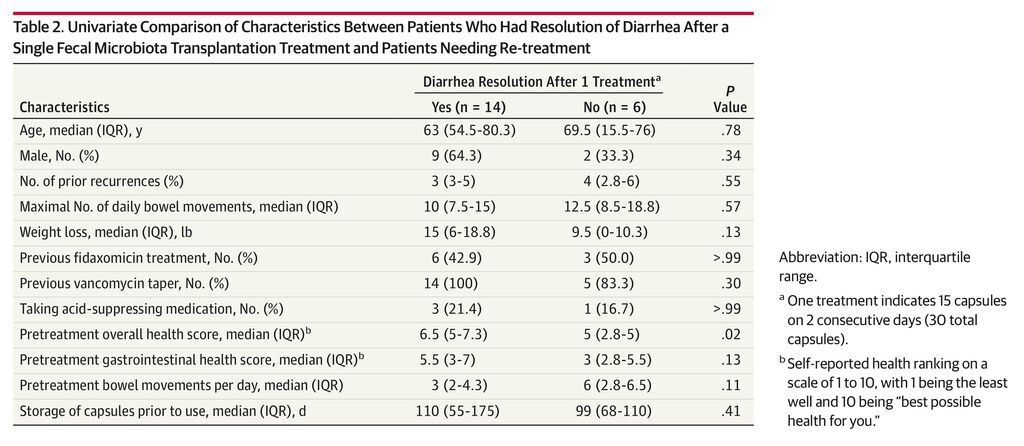
Secondary Outcomes
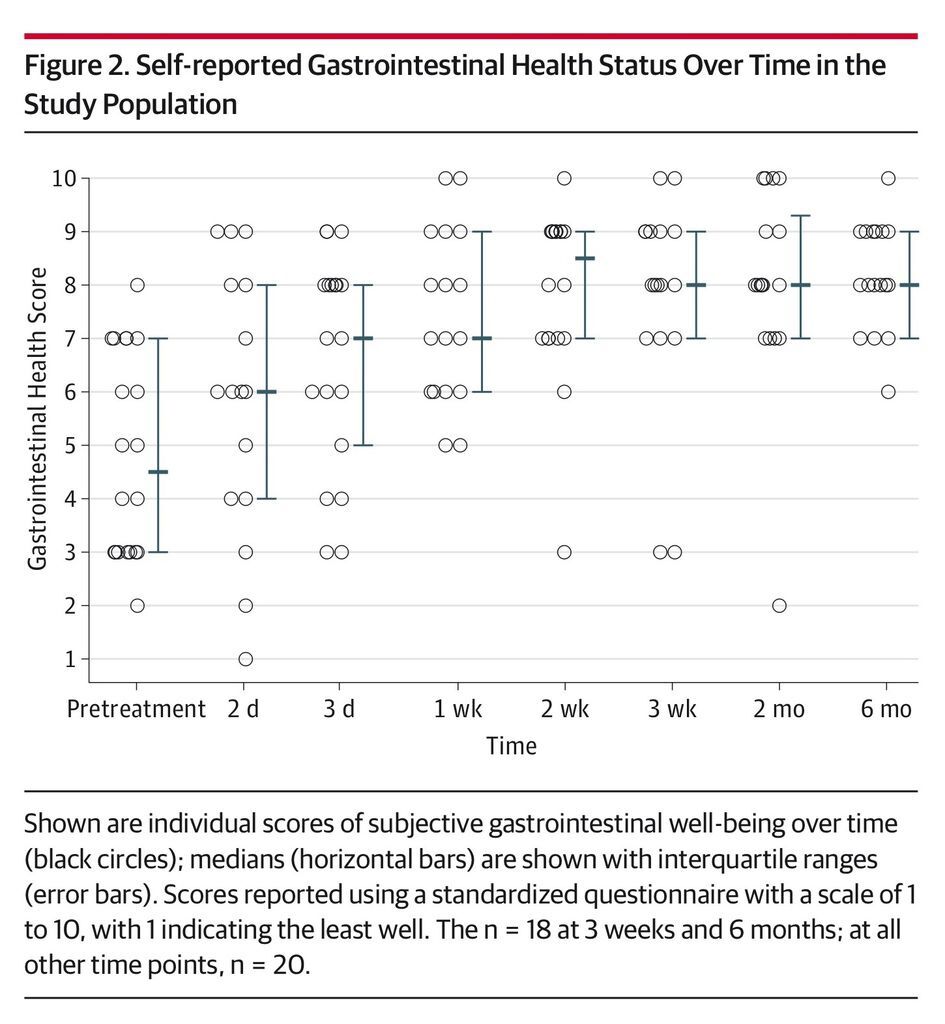
Daily number of bowel movements decreased from a median of 5 (IQR, 3-6) on the day prior to administration to 2 (IQR, 1-3) at day 3 (P = .001) and 1 (IQR, 1-2) at 8 weeks (P < .001) (Figure 1). Self-reported health rating using a standardized questionnaire scale of 1 to 10 improved significantly over the study period from a median of 5 (IQR, 5-7) for overall health and 4.5 (IQR, 3-7) for gastrointestinal health on the day prior to FMT to 8 (IQR, 7-9) at 8 weeks after the administration for both ratings (P = .001) (Figure 2 and Figure 3). A linear mixed model controlling for donor clustering found no association of age, sex, number of previous recurrences, previous vancomycin taper or fidaxomicin treatment, maximal number of bowel movements, acid-suppressing treatment, and storage time with any secondary outcomes (eTable 2 in the Supplement).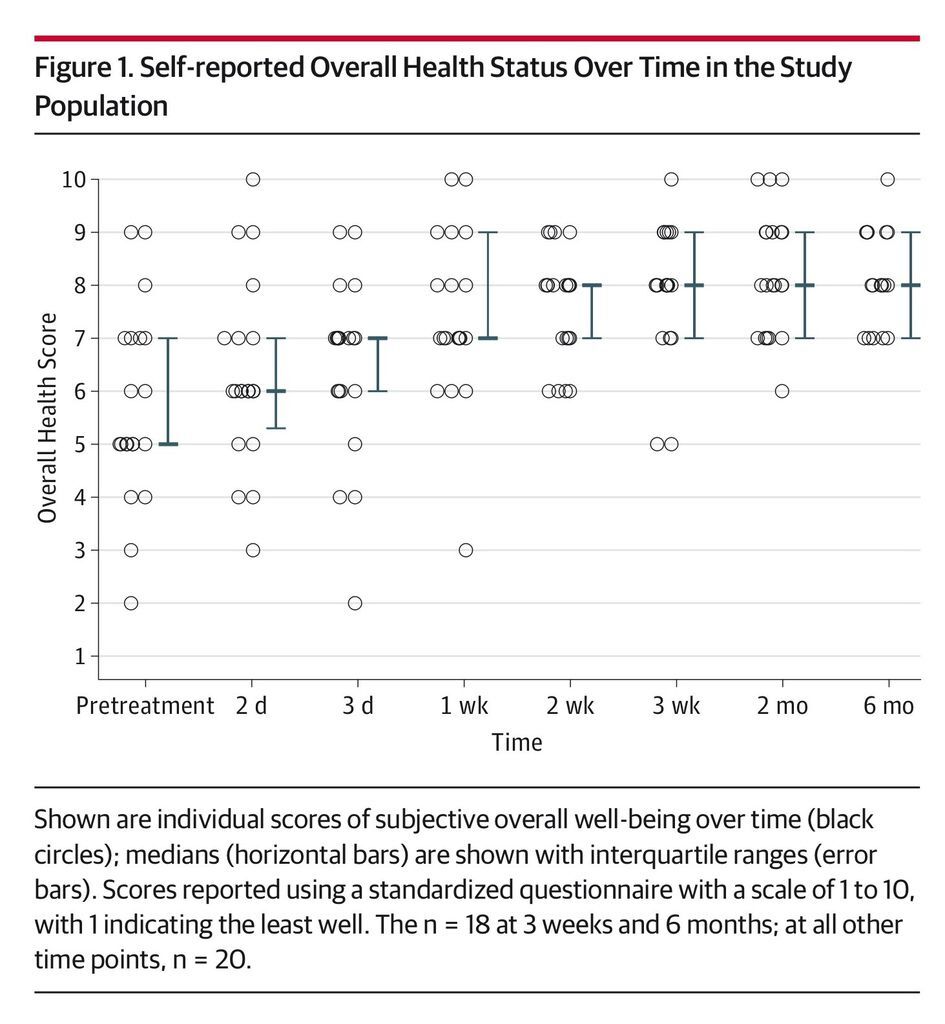
No patient vomited within 24 hours of capsule administration. Mild adverse events deemed likely related included posttreatment abdominal cramping and bloating in 6 patients (30%). In all cases, these problems resolved within 72 hours. One patient was hospitalized with a documented relapse of severe CDI after taking 15 capsules but had successful treatment after receiving the remaining 15 capsules after discharge. Additional clinical information about study patients can be found in eTable 3 in the Supplement.
In this preliminary study, we demonstrated the feasibility of oral administration of frozen encapsulated fecal material from unrelated donors to treat patients with recurrent CDI, with an overall rate of clinical resolution of diarrhea of 90%. These data are comparable with rates reported previously in case series and 1 randomized clinical trial using fresh stool preparations.13 We recently reported our experience with colonoscopic or nasogastric tube administration of frozen liquefied inocula15; the capsules achieved similar rates of diarrhea resolution (18/20 [90%] in both studies; P > .99). Time to resolution of symptoms was longer in the current study (mean, 4 days [SD, 1.9 days]) than when the inoculum was administered with colonoscope or nasogastric tube (mean, 2 days [SD, 0.8 days]; P = .03).
If reproduced in future studies with active controls, these results may help make FMT accessible to a wider population of patients, in addition to potentially making the procedure safer. The use of frozen inocula allows for screening of donors in advance. Furthermore, storage of frozen material allows retesting of donors for possible incubating viral infections prior to administration. The use of capsules obviates the need for invasive procedures for administration, further increasing the safety of FMT by avoiding procedure-associated complications and significantly reducing cost. A recent study found colonoscopic administration of FMT to be the most cost-effective treatment strategy for recurrent CDI compared with vancomycin or fidaxomicin.25 It is possible that with oral administration, assuming similar clinical outcomes, these results may be even more favorable.
The main limitations of this study are the small sample size and lack of placebo or active comparator. Although we report an overall rate of clinical resolution of diarrhea of 90%, the 95% CI is wide (68%-98%), most likely due to the small cohort size. The observed rate of resolution of diarrhea, however, is compatible with previous reports of randomized and observational studies evaluating other modes of delivery for FMT in patients with CDI.13,15 In most instances, the patients treated had baseline poor health, multiple prior standard antibiotic courses for CDI had failed, and FMT was available clinically in the catchment area. Based on the available data regarding efficacy of FMT and relapse rates with standard antimicrobial therapy, we elected not to perform a placebo-controlled or active standard-treatment comparator trial.
Some safety concerns remain. Vomiting and aspiration are potential concerns with oral delivery of FMT, although we did not observe these complications in our study. Even with careful screening, transmission of infection is possible but was also not observed in our patients. Also, the long-term effects of microbiota manipulation are still unclear, especially in light of the increasingly recognized roles of the human gut microbiome in health and disease. These potential risks should be weighed against the significant morbidity and mortality associated with recurrent CDI and the rates of diarrhea resolution reported following FMT administration.
The results of this preliminary study among patients with relapsing CDI provide data on adverse events and rates of resolution of diarrhea following administration of FMT using frozen encapsulated inoculum from unrelated donors. Larger studies are needed to confirm these results and to evaluate long-term safety and effectiveness.






 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 