Overview
FDA Approval: Adult, no; Pediatric, no
Efficacy: Adult, Evidence is inconclusive
Recommendation: Adult, Class III
Strength of Evidence: Adult, Category B
See Drug Consult reference: "RECOMMENDATION AND EVIDENCE RATINGS"
b) Summary:
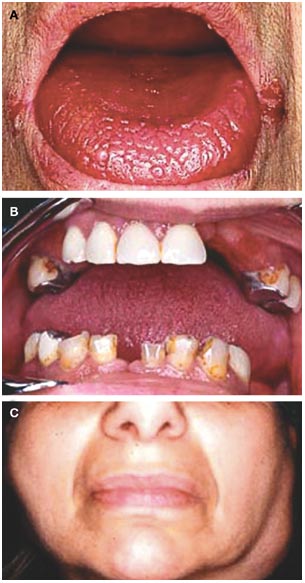
Enhanced lacrimal secretion has been observed in patients with Sjogren's syndrome in some controlled studies but not others
c) Adult:
1) An improvement in ophthalmological tests indicative of enhanced lacrimal secretion has been observed in patients with Sjögren's syndrome during oral BROMHEXINE therapy in some controlled studies but not others; subjective improvement was not reported by the patient in any of these studies, nor was the rate of tear secretion in healthy subjects affected by bromhexine (Avisar et al, 1996b). AMBROXOL, a metabolite of BROMHEXINE, has not been effective in producing objective improvement; additional studies are needed to clarify the role of BROMHEXINE in Sjögren's syndrome.
2) Two placebo-controlled studies have reported the efficacy of BROMHEXINE 16 milligrams orally three times daily in stimulating tear production in patients with Sjögren's syndrome (Manthorpe et al, 1981; Frost-Larsen et al, 1978a). In both trials, Schirmer test and break-up time (wetting time) values were higher after BROMHEXINE treatment as compared to placebo. However, no subjective improvement with regard to dryness of the eyes or mouth was reported by the patient in these studies. One other randomized study (Tapper-Jones et al, 1980a) reported no change in either Schirmer test values or lacrimal lysozyme concentrations in comparison with placebo in patients with Sjögren's syndrome. Salivary flow rates increased with both placebo and BROMHEXINE, with a trend toward greater improvement in this parameter with placebo. BROMHEXINE was not associated with subjective improvement.
3) Early studies employed relatively small numbers of patients, and 2 of the studies (Tapper-Jones et al, 1980a; Frost-Larsen et al, 1978a) have been criticized for possibly incorporating patients not meeting the diagnostic criteria for Sjögren's syndrome (eg, combined presence of keratoconjunctivitis sicca, a connective tissue disorder, and xerostomia) (Mackie & Seal, 1978; Manthorpe et al, 1980).
4) A metabolite of BROMHEXINE, AMBROXOL (NA-872), has also been investigated in the treatment of Sjögren's syndrome. It was speculated that this metabolite may be partly or wholly responsible for exocrine stimulatory effects during BROMHEXINE administration (Manthorpe & Prause, 1986a; Manthorpe et al, 1984a). However, AMBROXOL 60 milligrams daily was ineffective in improving objective ophthalmological tests in 1 controlled study involving 36 patients with Sjögren's syndrome (Manthorpe et al, 1984a).
另外,Pilocarpine也可以用在Sjögren's syndrome (修格連氏症候群)
另外,Pilocarpine也可以用在Sjögren's syndrome (修格連氏症候群)
全站熱搜





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 