Investigators have selected the first drugs to be evaluated in an Alzheimer's disease (AD) prevention trial.
All 3 agents target beta amyloid and will be used in a trial conducted by the Dominantly Inherited Alzheimer's Network Trials Unit (DIAN TU) at Washington University School of Medicine in St. Louis.
Researchers chose the selected agents from more than a dozen nominations submitted by the DIAN Pharma Consortium, which is composed of 10 pharmaceutical companies.
Each of the 3 drugs has been tested in previous trials for safety and efficacy. They include the following:
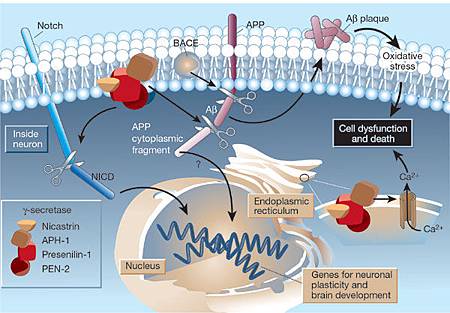
- Gantenerumab (Roche), a monocolonal antibody that binds to all forms of aggregated beta amyloid. It is currently being tested in an international phase II/III trial in prodromal AD.
- Solanezumab (Eli Lilly & Co), a monoclonal antibody that binds to soluble forms of beta amyloid. It is in phase III clinical trials.
- The BACE inhibitor, also from Eli Lilly, targets beta amyloid, theoretically reducing the production of beta amyloid and slowing the accumulation of plaques in the brain.
Funded by the National Institutes of Health, the DIAN trial, which is expected to start by early 2013, will include individuals who are mutation carriers (MCs) for autosomal-dominant AD and therefore are genetically destined to develop the rare and aggressive form of the disease at a young age, typically when they are in their 30s, 40s, or 50s.
The trial will involve 160 individuals, 80 MCs and 80 individuals who are DIAN participants but not MCs.
MCs will be randomly assigned to receive one of the 3 investigational drugs or placebo. Non-MCs will receive placebo.
"Normally in clinical trials there is a 50/50 chance of receiving the active drug or a placebo, but the efficient design of testing 3 drugs will allow us to significantly boost the number of participants who receive active treatment," principal investigator Randall Bateman, MD, said in a statement.
All individuals in the active treatment group will be within 10 to 15 years of becoming symptomatic. The first results of the DIAN study were published online July 11 in the New England Journal of Medicine and were reported by Medscape Medical News at that time. They showed that MCs have measurable biochemical and imaging markers, including changes in cerebrospinal fluid, brain amyloid deposition, and brain metabolism, that can be detected up to 25 years before AD symptoms appear.
These biomarkers will be monitored to determine whether the treatments stop or slow the disease process. The first phase of the study will last 2 years. It will be expanded and extended if 1 or more of the agents are shown to be effective.
For more information or to register for potential participation in the DIAN trial, contact www.dianxr.org or call 1-800-747-2979.
廣告打的很大,到時候失敗就不好看了.......





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 