- Nature Medicine Volume:16,Pages:1194–1195 Year published: (2010) DOI: doi:10.1038/nm1110-1194 Published online 04 November 2010
In Crohn's disease, immune damage to the gut wall is both induced and
modified by the gut microflora, challenging researchers to solve the
maze of interactions exploitable for therapeutic benefit. Whether these
microbial 'guests' are worsening or helping in this scenario is still
open to debate. In 'Bench to Bedside', Warren Strober highlights mice
studies showing that certain microbes in the gut have a protective role
promoting a shift towards an increased regulatory response that protects
from recurrence of the disease. In 'Bedside to Bench', Thomas MacDonald
examines how human studies using strategies to block soluble
proinflammatory cytokines—despite solid supporting data from animal
models—have shown disappointing results compared with therapies that
neutralize soluble cytokines but also deplete proinflammatory cells,
calling into question whether targeting a single soluble cytokine will
ever be useful to treat people with Crohn's disease.
Bedside to Bench
Companies such as PDL Biopharma and Centocor, which make fontoluzimab, a humanized interferon-γ (IFN-γ)-specific antibody, and ustekinumab, the fully human antibody specific for interleukin-12 (IL-12) and IL-23 p40, respectively, expected their chances of success in Crohn's disease to be high.
Crohn's disease is a condition almost certainly caused by an excessive T cell–mediated immune response in the gut wall against the antigens of the microbial flora. This T cell response seems to have all the hallmarks of classical T helper type 1 (TH1) bias—an abundance of the TH1-inducing cytokine IL-12, activated signal transducer and activator of transcription-4 and overexpression of T-bet (the transcription factor that promotes the production of IFN- γ) in mucosal T cells, increased mucosal IFN-γ protein abundance and vigorous IFN-γ production by Crohn's disease T cells in vitro1. The rationale, then, to test fontolizumab and ustekinumab in Crohn's disease was strong; negative clinical trials, however, have dampened the hopes of researchers, physicians and patients.
An early study showed that a single infusion of fontolizumab at 4 mg per kg body weight or even 10 mg per kg body weight was not superior to placebo in inducing a clinical response at day 28 in individuals with moderate to severe Crohn's disease2. In another study with fontoluzimab, in which different doses were infused (0.1, 1 and 4 mg per kg body weight), no difference in remission rates or response rates compared to placebo were seen at days 15 and 29 after treatment3. Finally, in a recent study where subjects received 1 or 4 mg per kg body weight of fontoluzimab intravenously, similar response rates were observed in subjects treated with the drug compared to placebo at day 29 (ref. 4).
The clinical trial of ustekinumab had a complex crossover design5. In the most crucial part of the study, individuals received placebo or 90 mg ustekinumab subcutaneously four times during the course of four weeks or 4.5 mg per kg body weight of the drug or placebo intravenously at the first day of treatment. Analysis of the clinical improvement and remission rates at weeks 4, 6 and 8 showed no difference of drug over placebo. The subjects were then crossed over at week 8 so that those given placebo in the early part of the study received ustekinumab, and vice versa. At weeks 12, 14 and 16, there were similar response and remission rates between the groups. There was, however, some slight evidence of greater efficacy of ustekinumab in patients who had previously received anti-TNF therapy. These results are consistent with an earlier phase 2 study of another IL-12 p40–specific antibody in active Crohn's disease, which suggested some efficacy but largely failed to achieve statistically significant differences between treatment and placebo, perhaps owing to a small group size6.
To put these results into context, people with active Crohn's disease treated with licensed tumor necrosis factor-α (TNF-α) antibodies, such as Remicade or Humira—the treatments of choice for complicated cases—usually have a 50% chance of achieving remission by days 28–42, a stunning result given that these individuals have usually failed to respond to steroids and the immunosuppressant azathioprione. Furthermore, as experience with TNF-targeting agents has been gained, studies such as SONIC, in which subjects received a combination of azathioprione and the TNF-α–specific antibody infliximab early in the course of disease, produce steroid-free remission in over 50% of people at week 26 and week 50 (ref. 7). So why were the therapeutic strategies with fontoluzimab and ustekinumab unsuccessful in clinical trials?
The obvious riposte to the disappointing results obtained with fontolizumab is that the antibody was directed at the wrong target; we now know that in Crohn's disease there is abundant production of another cytokine, IL-17A, which promotes neutrophil infiltration into tissues8. But this does not bear scrutiny, as human TH17 cells, which produce IL-17A, are highly dependent on IL-23, a cytokine overexpressed in Crohn's disease. Given that IL-23 shares a common subunit with IL-12, the human trials of antibody to IL-12 p40 also effectively neutralized IL-23, and therefore they should have decreased IL-17 production. The importance of IL-17 as a pro-inflammatory mediator was also not widely appreciated until 2006, by which time clinical studies neutralizing IFN-γ and IL-12 p40 were well underway. The same logic may indicate that IL-17 neutralization on its own should be also ineffective unless it is combined with fontolizumab, the antibody against IFN-γ.
Did the urge to block IFN-γ and IL-12 in Crohn's disease become more a triumph of hope over experience? In fact, it was the reverse—the rationale was, and is, compelling. The preclinical data from mouse models of inflammatory bowel disease (IBD) was strong for IL-12, although less so for IFN-γ, and the key molecules were overexpressed in diseased human tissue1. The failure of fontoluzimab and ustekinumab in clinical trials is therefore surprising and saddening. Yet it raises the issue as to whether blocking soluble cytokines is even worth pursuing in IBD.
These disappointing data are in line with a trend in clinical trials in IBD—nothing ever beats Remicade. All antibodies to TNF-α neutralize TNF-α, but Remicade and Humira have the added advantage that they also kill T cells and macrophages expressing membrane TNF-α, so they effectively deplete inflammatory cells. In addition, after binding membrane TNF-α, the drugs also block the production of other inflammatory cytokines such as IL-1β and IL-6 (ref. 9).
Agents such as Cimzia, a pegylated Fab2 that also blocks TNF-α, have shown positive results in clinical trials; overall, however, in my opinion, they seem not quite as effective as Remicade. Although Cimzia inhibits proinflammatory cytokine production, it does not deplete cells. Likewise, neutralization of IL-6, another cytokine shown to be involved in Crohn's disease10, was also not particularly effective11 and now seems to have been abandoned.
The general conclusion is that redundancy in the soluble cytokine pathways that maintain activated T cells in the gut in Crohn's disease, and the myriad of effector molecules damaging the gut, may make the targeting of a single soluble molecule a fruitless effort in people with Crohn's disease (Fig. 1). Unfortunately, this may mean reconsidering the use of mouse models of colitis in which many studies show inhibition of soluble cytokines to be a highly effective therapy12. Indeed, it is remarkable that so many diverse interventions worked in mice. This suggests that mucosal inflammation is so complex that any change in cytokine activity could have a positive effect in mice yet might not translate into clinical effectiveness in humans.
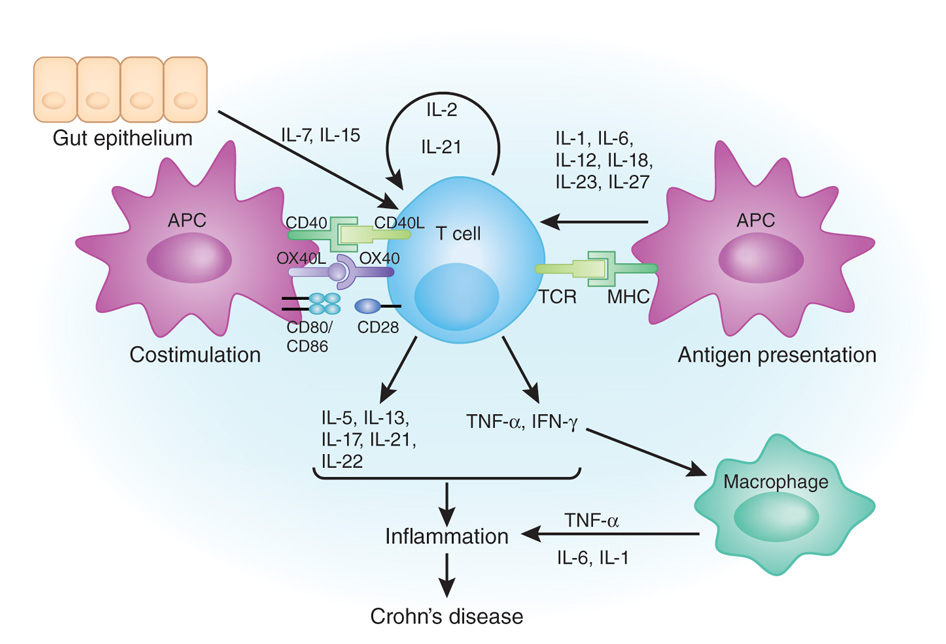
In Crohn's disease, proinflammatory CD4+ T cells are bombarded with survival signals. They may receive antigen-specific activation signals and co-stimulation signals from antigen-presenting cells (APCs). T cell secrete and receive signals from a plethora of cytokines—autocrine mediators which promote their survival, such as IL-2 and IL-21; signals from antigen-presenting cells, such as IL-1, IL-6, IL-12, IL-18, IL-23 and IL-27; and survival signals from gut epithelium, including IL-7 and IL-15. At the same time, they also can secrete many cytokines, which are generally proinflammatory, such as TNF-α, IFN-γ and IL-21. Agents aimed at blocking a single cytokine may not be very effective in Crohn's disease, as the proinflammatory landscape involves numerous signaling molecules and cell interactions.
The real lesson from the bedside to the bench—in light of the failure of fontoluzimab and ustekinumab in the treatment of Crohn's disease and alongside the success of infliximab and Humira—is that, to have a real effect, you have to kill or disable the enemy in the gut wall, the T cells and macrophages that cause IBD. The success of therapies with antibodies to TNF-α did not show that neutralizing cytokines can be therapeutic in IBD; instead, it showed that if you kill or disable the origin of such cytokines, the proinflammatory cells, the disease may go away.
Nevertheless, why Humira and Remicade only work in 50% of people with Crohn's disease will still require further investigation, as, in reality, very little is known about the interactions between therapeutic antibodies and immune cells in the inflamed human gut.





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 