
The US Food and Drug Administration (FDA) has approved fentanyl nasal spray (Lazanda) for the management of breakthrough pain in adults with cancer. It is indicated for patients aged 18 years and older who are already receiving opioid therapy but who have developed resistance to their regimen.
Fentanyl nasal spray is already available in 5 European countries, where it is marketed as PecFent (fentanyl pectin nasal spray).
"Lazanda is an important new option for patients with cancer who experience excruciating breakthrough pain," said Jeffrey H. Buchalter, chief executive officer of Archimedes Pharma, the manufacturer of the drug.
"Lazanda, which uses our patented PecSys drug delivery system, is designed to deliver medicine in a rapid, but controlled manner, and provides patients with an effective alternative to manage their breakthrough pain," he added in a statement.
Fentanyl is a schedule II controlled substance. The nasal spray device delivers fentanyl as a fine mist to the mucus membrane, according to the company. Each spray forms a gel when it comes in contact with the nasal mucosa, and then the active ingredient is absorbed across the mucus membrane and into the blood stream.
Efficacy Established
Efficacy was established in a double-blind, placebo-controlled clinical study of more than 500 opioid-tolerant adults with cancer and breakthrough pain. The clinical trial included an open-label titration phase, and then a double-blind placebo controlled phase, in which patients whose dose was titrated to an adequate level were randomly assigned to a blinded sequence of 10 treatments.
The primary outcome measure of the study was the mean sum of the pain intensity difference at 30 minutes. The difference was statistically significantly higher for the patients receiving fentanyl nasal spray than for those receiving placebo.
The most common adverse events associated with its use were consistent with opioid treatment and included vomiting, nausea, pyrexia, and constipation.
This formulation is indicated only for those with breakthrough pain and who are tolerant to opioid therapy. Patients who are considered opioid tolerant are those who are taking at least 60 mg of oral morphine per day, 25 mcg of transdermal fentanyl per hour, 30 mg of oral oxycodone/day, 8 mg of oral hydromorphone per day, 25 mg of oral oxymorphone per day, or an equianalgesic dose of another opioid for a week or longer.
It is contraindicated in the management of pain in opioid nontolerant patients because life-threatening hypoventilation could occur at any dose in patients not already taking around-the-clock opioid therapy.
This specific formulation is also not equivalent to other fentanyl products that are used to treat breakthrough pain. There are differences in the pharmacokinetics, which could potentially result in clinically important differences in the amount of fentanyl absorbed, possibly resulting in a fatal overdose.
Use in Specific Populations
The safety and efficacy in patients younger than 18 years of age have not been established. The drug is category C in pregnancy, meaning that there are no adequate and well-controlled studies of the drug in pregnant women. The drug should be used in pregnancy only if the potential benefit justifies the potential risk to the fetus, and it should not be used during labor and delivery or in women who are nursing.
It should be administered with caution in patients with impaired renal or hepatic function and titrated to clinical effect in patients with severe renal or hepatic disease.
REMS Required
According to the manufacturer's press release, the nasal spray formulation will become available in the second half of this year via a Risk Evaluation and Mitigation Strategy (REMS) program. This is intended to minimize the risk for misuse, abuse, addiction, overdose, and serious complications due to medication errors. Pharmacies, distributors, and healthcare professionals who prescribe to outpatients are required to enroll in the REMS program to dispense, distribute, and prescribe the drug.
如果你對這間公司有點印象,應該會對他在今年一月發表的新產品不陌生:PacFent

其實他也是fentayl鼻用噴劑
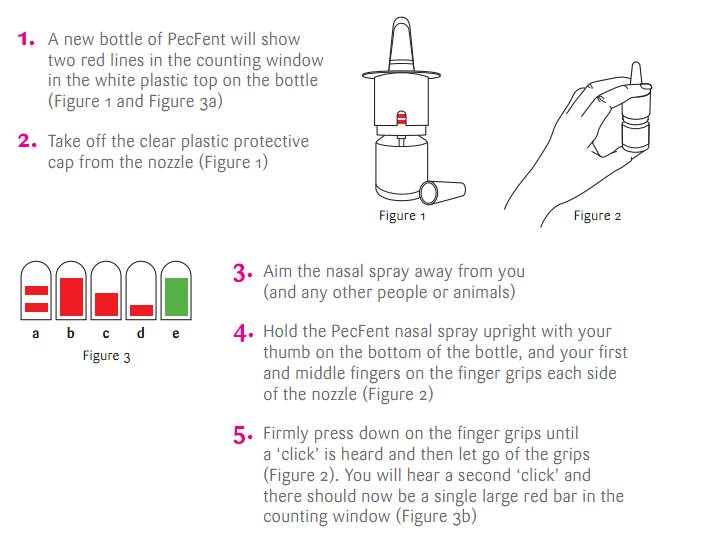
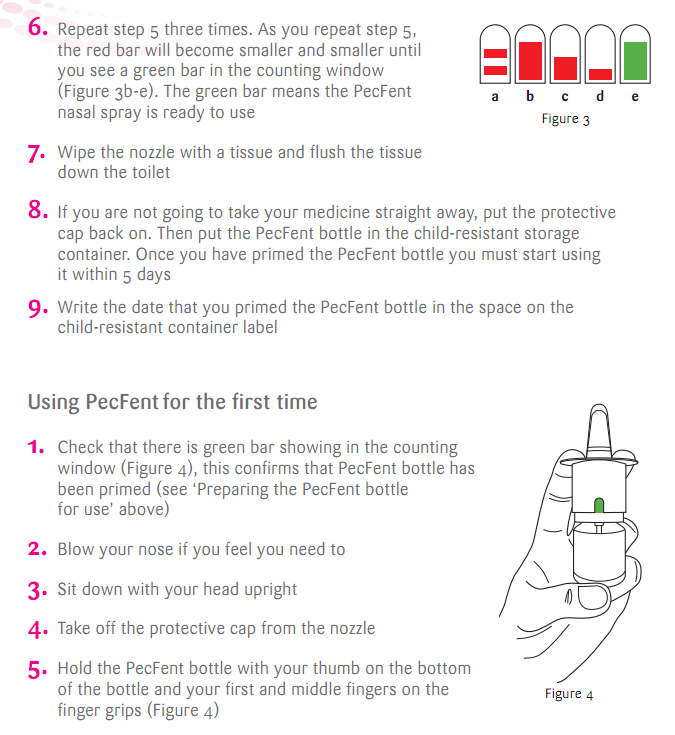
在這邊附上一點使用方法:
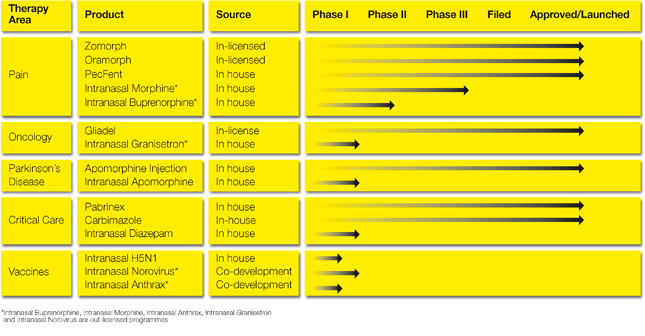
最後,你可以從他公司的研究期程中發現,他還有morphine、Apomorphine、granisetron、疫苗的鼻噴劑在開發中








 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 