藥師應該對幹細胞移植知道什麼呢?
Editor's Note:
Hematopoietic stem cell transplantation (HSCT) provides the only
potential curative option for many patients with hematologic
malignancies and is a widely used modality in relapsed or aggressive
disease. The strategy for this procedure is to replace all of the
diseased hematopoietic precursor cells with healthy ones, either by
using the patient's own hematopoietic stem cells once a complete
response with treatment is achieved (autologous) or by using healthy
stem cells from a donor (allogeneic). The bone marrow must be completely
ablated to remove any residual cancer precursor cells prior to infusion
of the healthy cells. To do this, a myeloablative procedure with
alkylating agents and total body radiation is used. Alternatively, a
reduced-intensity conditioning or nonmyeloablative procedure can be
used, but with this type of regimen the burden of ridding the marrow of
cancer cells rests on a graft-vs-malignancy effect. Myeloablation is a
relatively toxic protocol in which drug concentrations must be carefully
monitored to ablate the bone marrow without causing end organ toxicity.
Because it is associated with higher rates of transplant-related
mortality it is limited to younger patients lacking comorbidities and
with good performance status. Reduced-intensity conditioning was
heralded as a means to limit toxicity after HSCT, especially for the
older patient. The aim was to promote the inherent antileukemic activity
of the transplant while reducing toxicity and transplant-related
mortality. More than 10 years on, much has been learned about the role
of conditioning in determining outcomes after transplantation. Although
the use of reduced-intensity conditioning as a preparative regimen has
increased the opportunity for a number of patients to benefit from HSCT,
the toxicity from graft-vs-host disease (GVHD) is equally potent in
causing failure and disease progression.
Autologous transplant has fewer complications because the cells are recognized as self. In some cases, posttransplant prophylaxis is required to remove residual disease. In allogeneic transplantation, finding a suitably matched donor in a timely manner is a challenge. Often, partially HLA-mismatched (HLA-haploidentical) stem cells from a related donor are used. In other cases, a partially matched unrelated donor, or cord blood as a source, is used. Historically, HLA-haploidentical HSCT has been considered extremely high risk due to high rates of life-threatening GVHD and nonrelapse mortality. However, research has led to methods of reducing GVHD, either by prophylaxis or by grafts that are manipulated to reduce activated and alloreactive T-cell content to reduce the incidence of GVHD.
This video lecture uses busulfan as an example of careful monitoring of the conditioning cocktail and presents a treatment paradigm for limiting end organ toxicity or failure and disease progression. Busulfan has been used in myeloablative protocols with total body radiation and in reduced-intensity conditioning in combination with fludarabine. It has been well studied in regard to pharmacokinetics and pharmacodynamics to achieve sufficient serum levels to ablate marrow without causing graft failure or end organ toxicities. This type of careful monitoring is required for all pre- and posttransplant cocktails to ensure patient safety and a successful transplant outcome.

Timothy Madden, PharmD: Hello. I'm Dr. Timothy Madden, Associate Professor of Cancer Medicine and Pharmacology and Director of the Pharmaceutical Development Center at the University of Texas MD Anderson Cancer Center in Houston, Texas. I'd like to welcome you to this Medscape CPE video lecture discussion, What Pharmacists Should Know About Stem Cell Transplantation.

The program goal is to review optimum administration of conditioning regimens based on pharmacokinetic variability of dosing and how they affect outcome.

Stem cell transplantation has become increasingly more popular since its introduction in 1970. There are a variety of transplant regimens now available. Previously, all were of the type that completely ablated marrow. There are now less intensive regimens, and the pharmacist needs to know the drugs used and their effects.

The indications for stem cell transplantation are immunodeficiency or cancer, predominantly either lymphoid or hematologic cancers. The goals of stem cell transplantation are to eradicate the immune system plus or minus the cancer. In reality, we are going after the cancer but immune modulation is needed, especially for allografts.[1,2]
The procedure is to obtain bone marrow cell precursors -- that is, white cells, platelets, and red blood cells -- from patients themselves (autologous transplant) or from a donor -- a related, a matched, or even a matched, unrelated donor, called allogeneic transplant (alloSCT). The goal is to administer high doses of chemotherapy and/or radiation to kill the bone marrow cells and hopefully the cancer. Then intravenous (IV) stem cell infusions of the collected graft are seeded to the immune system and bone marrow by IV infusion and reinfusion of that collected bone marrow.

The pharmacokinetic goals of therapy for transplantation are very similar to this graph, and it is a double-edged sword. The idea is to deliver an ideal drug exposure where one might have myeloablation, cure of the cancer, and suppression of the immune system, while avoiding graft rejection, leukemic or disease progression, or, on the far side of the graft, organ toxicity. That's highly dependent on drug exposure. We all believe that individuals take a dose of drug and receive very similar outcomes with those drugs. It's very true for drugs like penicillin to have large therapeutic windows. And we often talk about a dose-response curve. But the reality is it's an exposure-response curve for most diseases.

This illustrates patients with a clearance that varies by 100% -- a patient having a clearance of 50 units vs 100 units. Receiving the same dose, one sees on the right hand side 2 different concentrations vs time curves. And we believe that translates into 2 different effect curves as well, where one ends up on one end of the spectrum or the other -- that is, having insufficient exposure to have an active clinical response vs having too high an exposure and having toxicity. We'd like to get somewhere in the middle. We have to do it in a population that has varying clearance.

One of the drugs used for myeloablative treatment is busulfan. It's an alkyl-alkane sulfonate and a bifunctional alkylator that is metabolized primarily by conjugation with glutathione. It was introduced over 50 years ago as a 2-mg tablet indicated for the palliative treatment of chronic myelogenous leukemia. At that time, little pharmacologic information was needed to be available to gain FDA approval.

In 1983, Dr. George Santos published his first paper, a seminal paper, in The New England Journal of Medicine looking at replacing total body irradiation (TBI), which had been one of the standards for myeloablation at the time, with busulfan. We'll get to those reasons in a moment. He used the dose (after many animal experiments) of 16 mg/kg every 6 hours for 16 doses orally and noted significant morbidity and mortality with this therapy. It was the only choice in dosing schema to come along at that point in time. Because the tablets are very small, 2 mg, a 100-kg patient would have to take 800 tablets per treatment course -- quite high.

However, it proved to be a very effective therapy. This slide demonstrates the superiority of the combination of cyclophosphamide and busulfan (BuCy2) vs cyclophosphamide and TBI (Cy/TBI) in patients with chronic myelogenous leukemia. The top curve shows the event-free survival, which is significantly better for the BuCy2 regimen through 5 years, as is the persistence of nonrelapse compared with the TBI group.

This is shown to be true with further studies in our institution, looking at TBI vs that combination of IV busulfan (the treatment in the previous slide being oral) and cyclophosphamide. In fact, there is a broader separation between the 2 regimens.

The mechanism of action of busulfan is that it damages DNA; it is an alkylating agent. It's available, as I said before, as 2-mg oral tablets or, since 1999, as a solubilized IV product in dimethyl acetamide. For the last decade, dosing has followed the Santos regimen of using oral tablets every 6 hours or IV every 6 hours for 16 doses.

Implications for therapeutic drug monitoring for busulfan suggest that these therapies are very high-dose therapies and have with them very high attendant complications. Likewise, failure to deliver a sufficient drug to a patient also jeopardizes the likelihood of long-term survival. So if concentrations are too low, one would have to deal with the immune wars, such as graft-vs-host disease (GVHD), graft failure for insufficient myeloablative therapy, and suboptimal anticancer effects. Concentrations that are too high result in hepatotoxicity and pulmonary and other toxicities that are often life-threatening.

This is a comparison slide of a single dose of IV vs oral busulfan looking at its pharmacokinetics over 6 hours. This is data taken from our laboratory. One can see that not only is there a definite lag -- the open white circles in the oral busulfan -- but that the shape and area under the concentration time curve (AUC)are quite dissimilar.

Some of the causes of altered bioavailability in this population result from nausea and vomiting associated with disease or even the ingestion of the oral drug, prior surgeries, radiotherapy or chemotherapy, changes in gut motility due to the use of antiemetics during the treatment period, and patient compliance (in ingesting adequate doses of drugs at the appropriate time).

In a study that we performed and published, we were able to demonstrate that there was greater than a 9-fold variance in oral bioavailability of the oral busulfan product, with some patients having as low as 10% bioavailability.

Compared with the previous erratic concentration vs time curve of the oral therapy to this IV busulfan therapy, also at 16 mg/kg per dose for 3 days, as one can see, it's regular and reproducible. In this particular case, this is a targeted protocol that uses the kinetics generated from these particular doses to target a patient to a specific daily AUC -- in this case, 4000 body surface area/kg AUC. As you can see through our pharmacokinetic intervention, we are within 2% of that value with an AUC of 3990 body surface area/kg.

The survival probability relative to busulfan AUC is noted in these plots from a study that we've published. It demonstrates that when one controls the exposure to some predetermined targeted AUCs with busulfan using an IV formulation -- in a way unachievable with an oral formulation -- we have very good relations to the probability of survival for 3, 6, 7, and 12 months. In fact, these curves point to AUCs that are related to the dose given to a specific patient based on their clearance.

In fact, in this large study of over 100 patients, the risk for death after bone marrow transplant relative to the busulfan AUC outlined for us a target interval for once-daily IV busulfan dosing of between 943 and 1300 micromolar x minute as an AUC. It's indicated as that slight dip in the curve, indicating that the probability of survival is best when that target is hit.

Likewise, when looking at Kaplan-Meier data based on that pharmacokinetic therapeutic interval study that was just shown, those individuals who had the AUC within the defined limit depicted by the top line fared far better over months than those who did not achieve that targeted AUC.
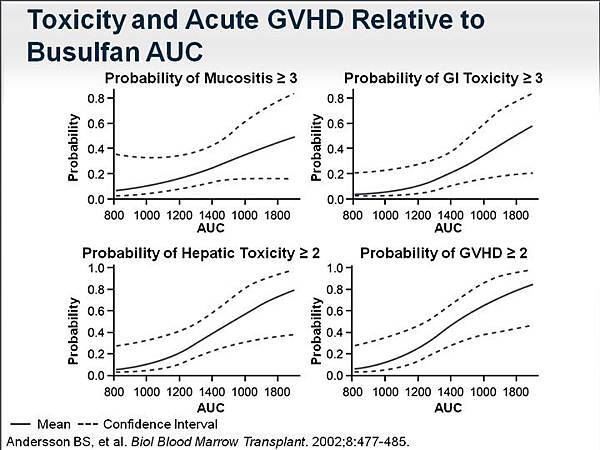
That target was related to toxicity as well. The probability of mucositis (grade ≥ 3), the probability of gastrointestinal (grade ≥ 3) and hepatic toxicity (grade ≥ 2), and the probability of GVHD (grade ≥ 2) were directly correlated to the exposure of busulfan. So it's clear that control of exposure to busulfan is extremely important as part of this regimen.

We have gone from using 4-times-daily IV busulfan to using once-daily busulfan for ease of use. We have conducted a number of studies that have demonstrated -- much like this from a patient at our institution -- the reliability of using pharmacokinetic monitoring and patient-specific dose adjusting to hit specific AUCs in protocols.

This is how it's done. An individual receives a starting dose. And patient characteristics, collected pharmacokinetic data, and response data to go into our model results in a pharmacokinetic model that describes for that individual patient what dose is needed based on their clearance to achieve what exposure. That's the control module. A revised dose is then administrated to the patient. The patient is remonitored to make sure that they hit those specific AUCs per dose for the entire treatment regimen. That is to say that if one were subdosed on dose 1, subsequent doses 2-4 are higher than just the target dose so that the entire therapeutic exposure can be achieved. By way of explanation, if 6000 AUCs is the target for a 4-day therapy, it's 24,000. So we would increase doses on days 2-4 based on previous data to achieve that entire course AUC.

There are issues of clinical concern with therapeutic drug monitoring for drugs that are used for myeloablative therapy. First, there are very few drugs for which there currently are assays that allow for patient monitoring. There are issues with quality of administered drug. The oral administered drug is one that has to be extemporaneously compounded by a pharmacist. That either means a suspension that's prepared in the pharmacy or the use of capsules to enclose the tablets, leading often to over- or underadministered doses as well as problems with patient emesis when busulfan is administered orally, then leading to the question of how much to readminister a patient who may have had an emetic episode. Sample collection procedures and documentation need to be well maintained. There are assay-related issues especially for smaller hospitals that do transplantation who simply don't have the means to do this type of monitoring. There are sites around the United States, however, that do rapid-turnaround analysis for busulfan in plasma and report values back to hospitals for dose adjustments. But someone needs to be able to do those calculations, and they are not by any means beyond the scope of what a pharmacist can do. And there are many areas where those individuals can get trained. There is the definition of targeted blood exposure, which has to be decided on ahead of time, and then the limitations of data and the recommendations that come from those data. That is to say, how frequently should we sample? When should we sample? How many samples should we obtain?

There are causes of altered drug disposition in cancer patients as well. They affect not only busulfan, but fludarabine, cyclophosphamide, and other drugs that are given and must be factored into the dosing -- things like weight loss or obesity. If the drugs are dosed on milligram per kilogram of body weight, there should be a correction factor for obese patients. There are issues of ascites or pleural effusions in those patients, which may lead to under- or overdosing given the distribution of the drug; and finally, hypoalbuminemia, which may be common in these patients and can affect the overall disposition.

Some of the causes of liver dysfunction in this patient population include metastases and decreased hepatic flow, especially in patients who have T-cell disease. Their liver can be highly impacted with white cells, leading to decreased hepatic blood flow. Often these patients have hepatitis, cirrhosis, or drug toxicity from previous therapies.

This leads to altered intrinsic hepatic clearances of the drugs that we're giving, reduced metabolic capacity, changes in important biliary excretion for many of these drugs, reduced carrier albumin production, and altered gastrointestinal absorption. The last point leads to a recommendation for the use of IV busulfan in this particular setting.

This leads to our ultimate therapeutic goal, successful myeloablation and transplantation. In order to do this, we have to achieve with whatever drug is used that therapeutic window that would ensure that not too low a dose was given, so that graft rejection and leukemia progression occurred, or that too high a dose was given so that end organ toxicity occurred.

It's a double-edged sword and a fine line to tread. It's true for all of the drugs that are used in this treatment, not just for busulfan -- so for cyclophosphamide, for fludarabine, for antiemetics, for the antibiotics, antifungals, immune modulators. The pharmacist should be responsible for monitoring organ function, body habitus, and drug metabolism and be responsible for individualizing therapy on all those agents as we have done for busulfan in this setting. I have included a supplemental readings list.

I'd like to thank you all for joining us for this Medscape CPE video lecture presentation today. Please continue on to the post-test to obtain CPE credit for this program.





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 