N Engl J Med 2005; 352:2314-2324June 2, 2005DOI: 10.1056/NEJMcp042803
This Journal feature begins with a case vignette highlighting a common clinical problem. Evidence supporting various strategies is then presented, followed by a review of formal guidelines, when they exist. The article ends with the author's clinical recommendations.
A 10-year-old girl with atopic dermatitis reports itching that has recently become relentless, resulting in sleep loss. Her mother has been reluctant to treat the girl with topical corticosteroids, because she was told that they damage the skin, but she is exhausted and wants relief for her child. How should the problem be managed?
THE CLINICAL PROBLEM
Atopic dermatitis (or atopic eczema) is an itchy, inflammatory skin condition with a predilection for the skin flexures.1 It is characterized by poorly defined erythema with edema, vesicles, and weeping in the acute stage and skin thickening (lichenification) in the chronic stage (FIGURE 1
Atopic Dermatitis. andFigure 1B).
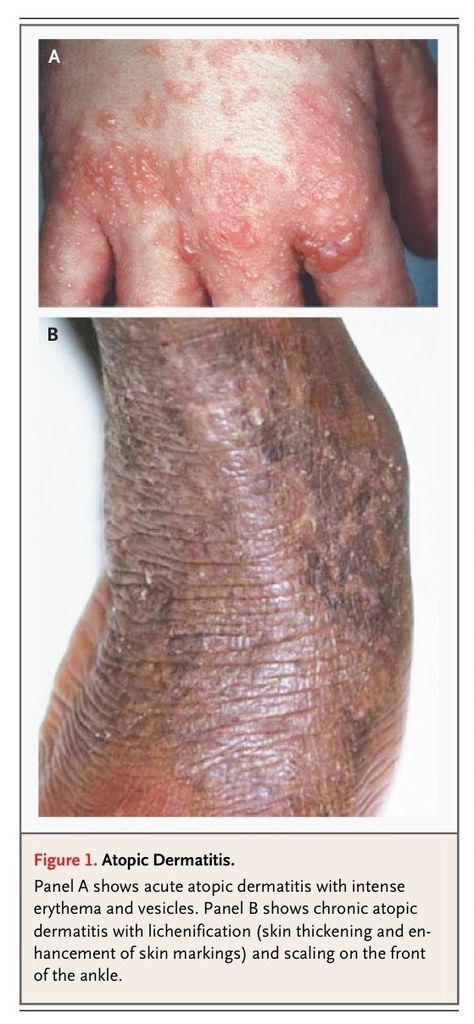
Although termed atopic, up to 60 percent of children with the clinical phenotype do not have demonstrable IgE-mediated sensitivity to allergens,2 an observation that led the World Allergy Organization to propose a revised nomenclature.3 Approximately 70 percent of cases of atopic dermatitis start in children under five years of age,4 although 10 percent of cases seen in hospital settings start in adults.5 Asthma develops in approximately 30 percent of children with atopic dermatitis, and allergic rhinitis in 35 percent.6
Diagnostic Criteria
Atopic dermatitis is difficult to define because of its variable morphology and distribution and its intermittent nature. Several diagnostic criteria have been developed.7 Consensus criteria for the main clinical features of atopic dermatitis8 have led to a short list of reliable and valid discriminators that are used worldwide9 (TABLE 1
Criteria for the Diagnosis of Atopic Dermatitis.).
Assessing disease severity is problematic when there is no objective marker.10 The many severity scales used in clinical trials are generally not suitable for rapid assessment in the clinic.11 The presence or absence of sleep disturbance, the number and location of involved sites, and the clinical course are the indicators of severity that probably provide the best basis for making decisions about treatment.12
Prevalence, Cost, and Prognosis
According to the International Study of Asthma and Allergies in Childhood, the prevalence of symptoms of atopic dermatitis in children six or seven years of age during a one-year period varied from less than 2 percent in Iran and China to approximately 20 percent in Australia, England, and Scandinavia.13 A high prevalence has also been found in the United States.14 In the United Kingdom, one population survey of 1760 affected children from one to five years of age found that 84 percent of cases were mild, 14 percent were moderate, and 2 percent were severe.15
Studies suggest that atopic dermatitis imposes a high economic burden,16 with out-of-pocket expenses and overall costs that are similar to those for the treatment of asthma.17 Causes of family stress related to caring for children with moderate or severe atopic dermatitis (e.g., sleep deprivation, loss of employment, time-consuming treatment, and financial costs) may rival those related to caring for children with diabetes mellitus type 1.18
Approximately 60 percent of patients with childhood atopic dermatitis are free of symptoms in early adolescence,19 although up to 50 percent may have recurrences in adulthood.20 Early-onset disease, severe early disease, concomitant asthma and hay fever, and a family history of atopic dermatitis may predict a more persistent course.4 One recent cohort study of 1314 German children showed that the prognosis was related to disease severity and atopic sensitization, as evidenced by elevated serum levels of IgE antibodies to food and inhalant allergens at two years of age.21
Causes
Atopic dermatitis is probably a complex disease relying on the interplay of several factors.22Several genes have been identified that may explain some cases.23 Genetics alone, however, cannot explain the results of studies of migrant populations that show, for example, that Jamaican children living in London are twice as likely to have atopic dermatitis as Jamaican children living in Jamaica; the increased risk of atopic dermatitis in smaller families and among higher social classes; and the rising prevalence of atopic dermatitis in some countries. These observations suggest a key role for the environment in mediating disease expression.24 Whereas allergens such as house-dust mites and foods may be important in some cases, nonallergic factors such as rough clothing, Staphylococcus aureus infections, exposure to microbes during infancy, excessive heat, and exposure to irritants that disrupt the function of the skin barrier may also be important. Mechanisms for and implications of the possible prevention of atopic dermatitis are reviewed elsewhere.25,26
STRATEGIES AND EVIDENCE
Diagnosis
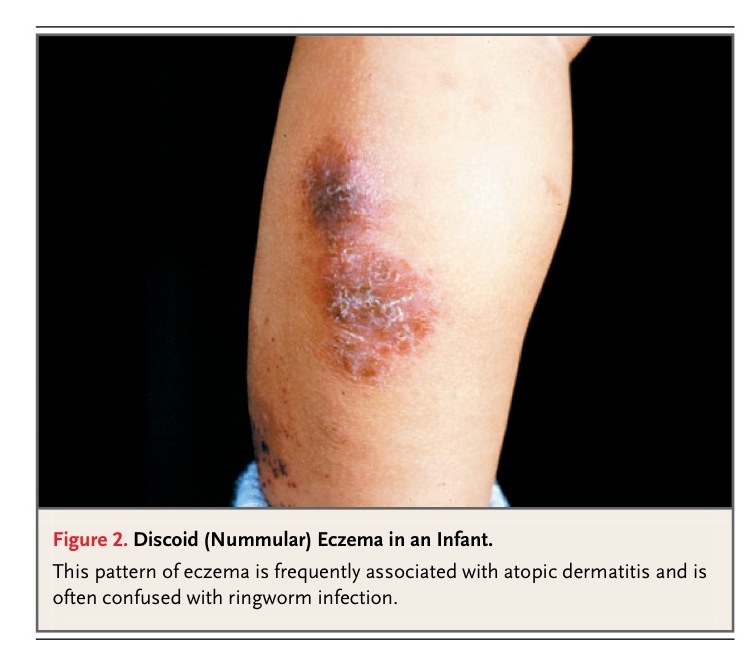
Skin biopsy is of little value in the diagnosis of atopic dermatitis; instead, diagnosis is based on clinical features7-9 (Table 1). The differential diagnosis depends on age and the country of residence (FIGURE 2
Discoid (Nummular) Eczema in an Infant. and TABLE 2
Differential Diagnosis of Atopic Dermatitis.). Because of their high negative predictive value (above 95 percent), negative skin-prick or radioallergosorbent tests for foods and environmental allergens may be useful for assessing the contribution of allergies to disease expression in children with severe disease.27 Positive tests are less useful, with positive predictive values of about 40 percent.27
Concomitant food allergy may be manifested as urticaria and gastrointestinal symptoms and may not necessarily exacerbate atopic dermatitis. Double-blind, placebo-controlled food challenges are the standard for diagnosing associated food allergy, but they are time consuming and not available in many hospitals.
The clinical utility of patch testing with airborne allergens is still unclear.28Patch tests are useful for excluding a diagnosis of suspected superimposed allergic contact dermatitis.29
Treatment
Topical Corticosteroids
One systematic review identified 83 randomized controlled trials of the use of topical corticosteroids in atopic dermatitis.30 Vehicle-controlled studies lasting less than one month indicate that approximately 80 percent of people report good, excellent, or clear responses with topical corticosteroids, whereas 38 percent of persons in control groups reported such responses.
Potency of topical corticosteroids is classified by the potential for vasoconstriction — a surrogate for clinical efficacy and skin thinning (TABLE 3
Therapeutic Interventions for Atopic Dermatitis.). In general, only preparations that have very weak or moderate strength are used on the face and genital area, whereas those that have moderate or potent strength are used on other areas of the body.31 Lower-potency corticosteroids may be sufficient on all areas of the body in younger children. Preparations are typically used in bursts of three to seven days in order to achieve control. There is little difference in outcome between short-term use of potent preparations or longer use of weaker preparations in children with mild-to-moderate disease.32 Lichenified atopic dermatitis requires more potent preparations for longer periods.
Long-term studies of moderate-to-potent preparations in children are scarce. One study of 231 children with stabilized atopic dermatitis randomly assigned to receive twice-weekly 0.05 percent fluticasone propionate (plus emollients) or vehicle alone plus emollients for 16 weeks showed that patients in the control group were more likely, by a factor of 8, to have a relapse (95 percent confidence interval, 4.3 to 15.2).33 A four-month trial of persons 12 to 64 years of age with moderate-to-severe disease showed that the application of fluticasone to previously active and new sites of atopic dermatitis for two consecutive days each week reduced flares significantly, as compared with a group receiving an emollient only.34
Reduced efficacy of topical corticosteroids may be related to disease severity rather than to glucocorticoid resistance.35 There is little evidence that the application of topical corticosteroids twice a day is more effective than once-daily applications,36 and more frequent use may cause more local side effects.
A main concern with the use of topical corticosteroids is irreversible skin thinning. Although thinning is possible, the concern on the part of patients (and parents) is often well out of proportion to the true risk.37 Although inappropriate use of potent preparations can cause skin thinning, four 16-week randomized trials did not show any clinically significant skin thinning,32-34,38 and a 1-year study showed no significant effect on collagen synthesis.39 A one-year study of unrestricted continual use of a potent corticosteroid on the limbs and trunk, a weak preparation on the face, or both showed that striae developed in 3 of 330 adults with moderate-to-severe atopic dermatitis.40Similar studies in children are lacking. Other possible side effects of corticosteroids include facial telangiectasia and glaucoma from periocular use (rarely reported in adults).
Secondary adrenal suppression and the suppression of growth resulting from systemic absorption of topical corticosteroids are also concerns, although clinically relevant adrenal suppression is very rare.41 One study involving children with atopic dermatitis did not find any relationship between height velocity and the use of mild-potency as compared with moderate-potency topical corticosteroids.42 Another study showed biochemical evidence of suppression of the hypothalamic–pituitary–adrenal axis only in children with atopic dermatitis who used potent or very potent topical corticosteroids and in those who had received glucocorticoids from other routes, and not in those who had used topical corticosteroids of mild or moderate strength for a median of 6.9 years.35
Emollients
There is no evidence that emollients improve atopic dermatitis directly. However, emollients are widely used because they improve the appearance and symptoms of the dry skin (xerosis) associated with this condition.30,31,43 One study has shown that emollients may reduce the need for topical corticosteroids by approximately 50 percent,44 and another study found that emollients enhanced the response to treatment with topical corticosteroids.45 There is little basis for suggesting the use of one emollient over another, and the preference of the patient is probably the most important factor.31
Topical Calcineurin Inhibitors
Topical tacrolimus and pimecrolimus have both been shown to be effective in vehicle-controlled studies. For 1 percent pimecrolimus, the rate ratio for the proportion of patients clear or almost clear of atopic dermatitis at three weeks in five vehicle-controlled trials involving 783 patients was 2.72 (95 percent confidence interval, 1.84 to 4.03).46 For 0.03 percent and 0.1 percent tacrolimus, the rate ratios for the proportion of patients who were clear or who had excellent improvement at 12 weeks were 4.50 (95 percent confidence interval, 2.91 to 6.96) and 5.62 (95 percent confidence interval, 3.67 to 8.61), respectively, in three vehicle-controlled trials involving 656 patients.46 Short-term studies suggest that 0.1 percent topical tacrolimus may be similar in strength to potent topical corticosteroids,46 whereas topical pimecrolimus is considerably weaker.40,47
Few long-term studies compare intermittent use of topical calcineurin inhibitors with intermittent use of topical corticosteroids. A 12-month vehicle-controlled study of children with atopic dermatitis showed that early use of pimecrolimus reduced the frequency of flares from 51 percent to 28 percent,48 although early use of mild topical corticosteroids might have shown similar effects.
Topical calcineurin inhibitors do not cause skin thinning. Both tacrolimus and pimecrolimus are associated with mild burning sensations when applied to the skin (Table 3). Five-year studies show a good safety profile for these agents.49 In the United Kingdom, the National Institute of Clinical Excellence approves the use of topical tacrolimus for children older than two years of age with moderate-to-severe atopic dermatitis not controlled by topical corticosteroids, and of topical pimecrolimus as a second-line option for resistant dermatitis of the head and neck.50 In the United States, both of these topical calcineurin inhibitors are approved as second-line agents, and the site of application is not restricted for pimecrolimus.
In March 2005, the Food and Drug Administration issued an alert to health care professionals concerning a potential link between topical pimecrolimus and tacrolimus and cancer (mainly lymphoma and skin cancer) on the basis of studies in animals, case reports, and knowledge of how these drugs work.51,52 The alert emphasizes the importance of using these preparations only as labeled and when first-line treatment has failed or cannot be tolerated.
Other Topical Agents
A study of a refined-coal cream used on one side of the body in adults with mild-to-moderate atopic dermatitis as compared with 1 percent hydrocortisone used on the other side suggested similar efficacy after four weeks.53 There is insufficient evidence to conclude whether topical cromoglycate preparations are effective.30,54 Other topical treatments — such as St. John's wort cream, vitamin B12, and licorice gel — whose use is supported by single small, randomized trials require further evaluation before they can be recommended for the treatment of atopic dermatitis.
Oral Antihistamines
Evidence is lacking to support the use of antihistamines for the treatment of atopic dermatitis,55although they are sometimes recommended for their sedative effects.56 Reports on nonsedative antihistamines are conflicting.30,56,57 The largest study failed to demonstrate any overall benefit from prolonged use of cetirizine in children with atopic dermatitis.58
Topical Doxepin
Topical doxepin produces some relief from itching within 48 hours. However, a clinically useful beneficial effect on disease severity has yet to be shown, and drowsiness may be a problem.30
Antibiotic Agents
Secondary infection with S. aureus is common (FIGURE 3
Acute, Secondary Infection in an Infant with Atopic Dermatitis.) and usually is treated with short courses of antibiotics such as floxacillin, cephalexin, or amoxicillin–clavulanate. One randomized trial found no benefit to prescribing floxacillin continually for four weeks as compared with placebo, and methicillin-resistant strains were more common in those who were prescribed antibiotics.59 Although combinations of topical corticosteroids and antibiotics are used for atopic dermatitis, no good evidence suggests that they offer additional benefits as compared with topical corticosteroids alone.30
Ultraviolet Light
Randomized clinical trials have shown that ultraviolet light (ultraviolet B, narrow-band ultraviolet B, and high-intensity ultraviolet A) is beneficial for atopic dermatitis in the short term.30 Burning and itching may occur, and carcinogenicity is a long-term concern. Phototherapy is usually used as a second- or third-line treatment.31
Immunosuppressive Agents
A brief course of oral corticosteroids (less than three weeks) may be used to control a flare of severe disease, although data from randomized clinical trials are lacking. Ongoing use of systemic immunosuppressive agents (oral corticosteroids, cyclosporine, azathioprine, mycophenolate, and interferon gamma) is limited by adverse effects and is usually reserved for people with severe disease who do not respond to other measures.30,43
Nonpharmacologic Approaches
Avoiding foods suspected to cause flares may be helpful in young children with severe disease, but usually is not helpful in adults.30,60 Little evidence supports dietary exclusion of milk and eggs in unselected cases.61 Some evidence supports egg-free diets in infants with atopic dermatitis who produce IgE antibodies to egg protein.60 No good evidence supports highly restrictive diets, which can sometimes cause malnutrition.62 Studies have failed to show clinically useful benefits from supplements such as evening primrose oil, borage oil,63 zinc, pyridoxine, or vitamin E,30 or from viable lactobacilli (probiotics).30,64
Small randomized trials support psychological approaches such as behavior therapy (to reduce the habit of scratching) and relaxation therapy.30 Parental-education programs and demonstration of topical treatments by caregivers may be helpful.65,66 Reduction of house-dust-mite allergen can reduce severity scores for atopic dermatitis, but the clinical relevance and sustainability of such reductions is unknown.30 Impermeable mattress covers are very effective in reducing levels of mite antigens, but they have no clear clinical benefit.67
No good evidence supports the use of bandages containing zinc paste. The use of “wet wraps” (an outer dry bandage overlying an inner damp bandage used over either emollients or topical corticosteroids) has become a popular second- or third-line measure for children with resistant atopic dermatitis but is not supported by randomized trials, and enhanced systemic absorption remains a concern.41 No good data support alternative or complementary therapies such as homeopathy and bioresonance.30
AREAS OF UNCERTAINTY
Randomized trials are lacking to assess the benefits of many simple interventions, such as emollients and other nonpharmacologic approaches.30 The lack of common outcome measures hinders meaningful comparisons across trials.11 Trials with active comparators are needed to inform choices among agents.47 Data on the optimal use of topical corticosteroids are needed, along with long-term data on adverse events. Data concerning the long-term safety of topical tacrolimus and pimecrolimus are also needed. The benefits of routine allergy testing require clarification. Moreover, it is unclear whether early aggressive therapy in children with atopic dermatitis alters the natural history of the disease.
GUIDELINES
The American Academy of Dermatology recently published evidence-based guidelines for atopic dermatitis that contain recommendations that are consistent with the evidence summarized in this article.43 In addition, many useful Web sites are available (TABLE 4
Web Sites with Information about Eczema.).
CONCLUSIONS AND RECOMMENDATIONS
Patients and families, such as the girl and her mother who are described in the vignette, often have concerns about topical corticosteroids that can be alleviated by appropriate education.68 Patients and families should be taught about the course of atopic dermatitis; that is, that a single cause and cure are unlikely, although good control is nearly always possible. Discussions should be supplemented by written information and a demonstration of the use of topical treatment.
For the girl in the vignette, I would recommend inducing a remission with once-daily application of a potent topical corticosteroid to the limbs and trunk for 10 days before scheduling a second visit to evaluate progress. Although data to support the use of emollients are limited, I would attempt to maintain remission by liberal use of emollients only, with recourse to five-day courses of potent or moderate-strength topical corticosteroids for flares.33 If such a regimen failed to maintain adequate quality of life, I would introduce “weekend therapy” — that is, the application of a potent corticosteroid to new and previously active sites of atopic dermatitis each Saturday and Sunday evening to reduce flares.34 Alternatively, intermittent use of topical tacrolimus or pimecrolimus may be used to reduce flares.50 If facial dermatitis requires continual use of mild topical corticosteroids, I would recommend the use of topical tacrolimus, 0.03 percent, twice daily for three weeks and then once daily until the atopic dermatitis clears up.50










 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 