Amne Borghol, PharmD, CGP
Clinical Associate Professor
Xavier University of Louisiana College of Pharmacy
Breannie Charles, PharmD Candidate
Xavier University of Louisiana College of Pharmacy
Fadi Hawawini, DO
Medical Director
Ochsner Extended Care Hospital/Ochsner Skilled Nursing Facility
New Orleans, Louisian
1/23/2013
US Pharm. 2013;38(1):23-26.
ABSTRACT: Migraine, a chronic neurologic disorder, involves episodic attacks of headache and associated symptoms. Although almost 26% of migraine patients fulfill criteria for preventive therapy, only one-half of sufferers use daily preventive medications. Triptans, antidepressants, antiepileptic medications, nonsteroidal anti-inflammatory drugs (NSAIDs), antiemetics, opioids, and cardiovascular agents have been investigated for migraine prophylaxis. In 2012, the American Academy of Neurology and the American Headache Society published updated evidence-based recommendations for episodic migraine prevention classified according to seven levels. Pharmacologic agents, NSAIDs, and complementary therapies are included in the revised guidelines. The guidelines also address new therapies for the short-term prevention of menstrually associated migraines, including frovatriptan, naratriptan, and zolmitriptan.
Migraine is a chronic neurologic disorder characterized by episodic attacks of headache and associated symptoms such as aura, nausea, vomiting, and photophobia. Extensive research has been conducted not only on how to treat migraine episodes, but also on how to prevent or reduce the number of occurrences. According to recent studies, nearly 26% of migraineurs meet the criteria for preventive therapy, but only 13% actually use daily preventive medications.1 Medications that have been investigated for migraine prophylaxis include nonsteroidal anti-inflammatory drugs (NSAIDs), triptans, antidepressants, opioids, antiepileptic drugs (AEDs), antiemetics, and cardiovascular medications.
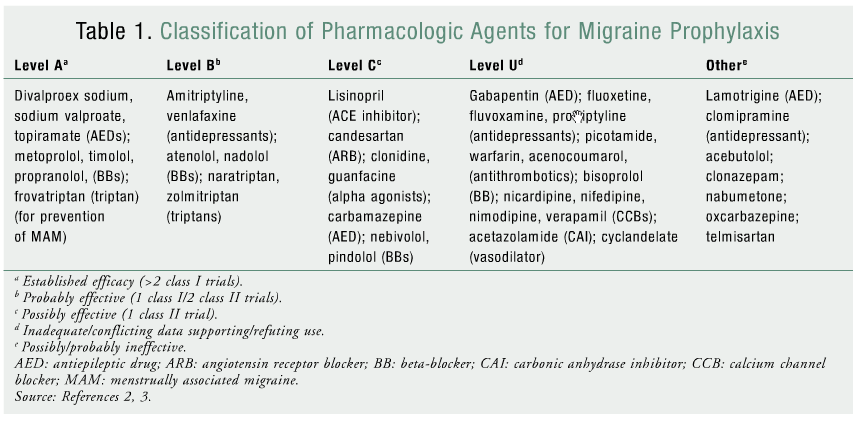
The American Academy of Neurology (AAN) published guidelines on episodic migraine prevention in adults in 2000.2 In 2012, the Quality Standards Subcommittee of the AAN and the American Headache Society (AHS) published revised evidence-based recommendations for episodic migraine prevention that upgraded or downgraded current pharmacologic agents and added new agents. Based on study results reviewed by the panel, pharmacologic agents were classified into seven recommendation categories (levels A, B, C, U, A negative, B negative, C negative) relative to their efficacy for migraine prophylaxis (TABLE 1).2
The new guidelines also included recommendations concerning the clinical efficacy of NSAIDs and complementary therapies (i.e., herbals, vitamins, minerals) for episodic migraine prophylaxis based on new evidence-based clinical trials.3 Treatments addressed were the NSAIDs fenoprofen, ibuprofen, ketoprofen, naproxen, and naproxen sodium; subcutaneous histamine; and the complementary therapies Petasites hybridus extract (butterbur), MIG-99 (feverfew), riboflavin (vitamin B), and magnesium.3
LEVEL A PHARMACOLOGIC AGENTS
Antiepileptic Drugs
Studies have suggested that the pathophysiology of migraine disorder may involve neuronal hyperexcitability, which has led to the introduction of AEDs for the treatment of episodic migraine based on their effects on gamma-aminobutyric acid, an inhibitory neurotransmitter.1
Divalproex Sodium and Sodium Valproate: Many trials have established the efficacy of divalproex sodium for migraine prophylaxis in terms of reduced migraine frequency, severity, and attacks. One randomized, double-blind, placebo-controlled, parallel-group study evaluated the efficacy, tolerability, and safety of an extended-release (XR) formulation of divalproex sodium as monotherapy for migraine prophylaxis.4 Of 237 subjects, 122 received the drug and 115 received placebo. The primary efficacy variable was a reduction from baseline in 4-week migraine rate.4
Overall, the divalproex sodium group had a mean reduction in headache rate of 1.2, versus 0.6 for the placebo group (P = .006). With regard to the reduction in number of migraine-episode days, the experimental-phase reduction from baseline (P = .009) was greater in the divalproex sodium group (mean value 1.7 vs. baseline mean value of 6.3) than in the placebo group (mean value 0.7 vs. baseline mean value of 5.8). The proportion of subjects achieving at least a 50% reduction in experimental-phase migraine rate was higher in the divalproex sodium group (30%) than in the placebo group (24%), but the difference was not significant (NS). There was no significant difference between groups in overall incidence of any specific adverse event (AE).4
Major findings of this study include the early-onset action of XR divalproex sodium in reducing headache frequency, days, and rate; the equivalent number of subjects in both groups who were removed from the study because of AEs; and the convenience of once-daily dosing for the XR formulation. The XR formulation of divalproex sodium is well tolerated and efficacious as monotherapy for migraine prophylaxis.4
Topiramate: Various studies have proven the effectiveness of high-dose (HD) topiramate in the prevention of episodic migraine. However, HD topiramate can yield significant AEs (e.g., fatigue, memory lapse, taste disturbances, weight loss, paresthesia), thereby reducing patient compliance.1
In an 8-week, randomized, double-blind clinical trial, investigators compared low-dose (LD) topiramate versus propranolol for migraine prophylaxis.1 The 62 patients received either topiramate 50 mg or propranolol 80 mg daily. Both drugs had significant efficacy with regard to frequency, intensity, and duration of migraine episodes. Topiramate achieved a higher mean reduction in frequency, intensity, and duration compared with propranolol; however, topiramate patients had slightly more severe baseline headache parameters, which may have contributed to the greater improvements. One topiramate patient and one propranolol patient withdrew because of severe paresthesia and hypotension intolerance, respectively.1 In another recent small study, topiramate helped reduce the number of migraine days per month, and it was concluded that topiramate is well tolerated and effective for the prevention of chronic migraine.5
Beta-Blockers (BBs)
Propranolol: Migraine-prophylaxis guidelines frequently recommend BBs as effective first-line therapy.1 Propranolol is one of the most commonly prescribed BBs for migraine prevention. Its efficacy has been well established in clinical trials comparing propranolol with placebo or with other drugs, such as topiramate.1
Metoprolol: The original AAN guidelines listed metoprolol as “probably effective” for migraine prophylaxis. More recently, metoprolol and acetylsalicylic acid (aspirin) were compared for migraine prophylaxis in a randomized, controlled, double-blind, parallel-group, phase III study.6 The objective was to evaluate the efficacy and safety of aspirin 300 mg versus metoprolol 200 mg. The primary efficacy endpoint was defined as a 50% reduction in the rate of migraine attacks. Aspirin treatment was administered to 135 patients; the remaining 135 received metoprolol. Metoprolol was more effective than aspirin in achieving 50% migraine-frequency reduction, with a 45% responder rate in the metoprolol group versus a 26% rate in the aspirin group.2,6 Migraine-attack frequency decreased from 3.55 to 1.82 in the metoprolol group and from 3.36 to 2.37 in the aspirin group.1 AEs were less frequent in the aspirin group than in the metoprolol group.5
Triptans
Frovatriptan: Frovatriptan, a new selective serotonin receptor agonist with a longer half-life than other triptans, is approved for short-term migraine prevention. Triptans have been evaluated for short-term prevention of menstrually associated migraine (MAM). MAM is usually more difficult to treat than regular migraine and has a longer duration.7 A double-blind, placebo-controlled, three-way crossover study assessed frovatriptan 2.5 mg as short-term preventive therapy for MAM.7 The primary efficacy endpoint was whether frovatriptan taken for 6 days (2 days before onset of menses to 4 days afterward) was more effective than placebo for MAM prevention. A secondary objective was to examine how well frovatriptan reduced MAM severity and duration. It was concluded that frovatriptan given prophylactically for 6 days during the perimenstrual period is highly effective for MAM prevention, severity reduction, and duration, which occurred in more than one-half of subjects. Frovatriptan also exhibited a well-tolerated AE profile.7
LEVEL B PHARMACOLOGIC AGENTS
Antidepressants
Venlafaxine and Amitriptyline: The efficacy and AEs of venlafaxine and amitriptyline in the prophylactic treatment of migraine were compared in a randomized, double-blind, crossover study.8 Patients, who underwent a 4-week run-in period with no prophylactic treatment, kept a headache diary noting the number, duration (hours), and severity of migraine attacks. Fifty-two of 76 patients completed the study. Five patients dropped out because of intolerable AEs associated with amitriptyline, and one dropped out because of AEs from venlafaxine. Both drugs were effective for migraine prophylaxis, but the difference between them was NS. Venlafaxine and amitriptyline were beneficial for pain parameters (P <.01) and achieved reductions in migraine frequency, intensity, and duration (P <.01). Although both drugs are effective, the AE profile of XR venlafaxine is much more favorable and tolerable than that of amitriptyline.8
Triptans
Naratriptan: The efficacy of naratriptan for short-term prevention of MAM during perimenstrual periods was examined in a randomized, double-blind, placebo-controlled study.9 Subjects took naratriptan 1 mg twice daily, 2.5 mg twice daily, or placebo twice daily. The primary efficacy endpoint was the number of MAMs occurring over four perimenstrual periods. It was concluded that naratriptan 1 mg twice daily is efficacious for preventive treatment of MAM and exhibits a well-tolerated AE profile. Although naratriptan 2.5 mg showed some traces of efficacy, the difference versus placebo was NS.9
Zolmitriptan: A randomized, placebo-controlled study examined the efficacy and safety of zolmitriptan in the short-term prevention of MAM.10 A total of 244 subjects were assigned to three treatment groups: zolmitriptan 2.5 mg orally twice daily (n = 80), zolmitriptan 2.5 mg orally three times daily (n = 83), and placebo three times daily (n = 81). Subjects, who were treated for three menstrual cycles, began therapy 2 days prior to onset of menses and continued after onset for a total of 7 days of treatment. The primary efficacy endpoint was a 50% or greater reduction in frequency of MAM attacks. Both zolmitriptan groups had efficacy superior to that of placebo in the prophylactic treatment of MAM.10
LEVEL A NEGATIVE PHARMACOLOGIC AGENTS
Lamotrigine
In a randomized, four-phase crossover study comparing the efficacy of LD topiramate and lamotrigine versus each other and placebo for migraine prophylaxis, topiramate was superior to placebo and lamotrigine, and lamotrigine was ineffective versus placebo and topiramate with regard to the primary efficacy endpoint (responder rate [50% reduction in mean migraine frequency and intensity]).11Sixty subjects who had frequent migraine attacks (>4 episodes/month) were randomized to lamotrigine 25 mg twice daily or matching placebo or to topiramate 25 mg twice daily or matching placebo. Subjects underwent treatment for 1 month, with a 7-day washout period, and recorded the frequency, severity, and symptoms of all headaches or auras.11
The responder rate for frequency was 46% in the lamotrigine group versus 34% in the placebo group (NS). The responder rate for headache intensity was 21% in the lamotrigine group versus 14% in the placebo group (NS). There was no statistically significant difference with regard to reduction in responder rate for migraine frequency or migraine intensity in the lamotrigine group versus the placebo group. Although lamotrigine was ineffective for migraine prophylaxis, the investigators commented that lamotrigine’s efficacy should not be automatically discounted, since the study duration was short and the statistical analysis power was not sufficient.11
NSAIDS
There have been no new clinical trials evaluating the efficacy of NSAIDs since the 2000 guidelines were published.3 Evidence continues to be conflicting regarding whether aspirin is efficacious for migraine prophylaxis; therefore, this agent remains a level U recommendation.3
COMPLEMENTARY THERAPIES
Level A Complementary Therapies
Petasites (Butterbur): Petasites plant extract has traditionally been used for migraine prophylaxis.11 In a double-blind, randomized, three-arm, parallel-group, placebo-controlled study, Petasites doses of 50 mg twice daily and 75 mg twice daily were compared with placebo twice daily to evaluate the herb’s efficacy in migraine prophylaxis. The primary efficacy endpoint was the change in frequency of migraine attacks per month over the 16-week treatment course.12 Petasites 75 mg twice daily was superior in efficacy to placebo and was statistically significant with regard to the primary efficacy endpoint. However, Petasites 50 mg twice daily did not show a statistically significant difference in reduction of headache frequency compared with placebo.
Level B Complementary Therapies
The revised guidelines list riboflavin, magnesium, and feverfew as complementary therapies that are “probably effective” for migraine prophylaxis, based on more recent clinical trials that investigated their efficacy.
Magnesium, Riboflavin, and Feverfew: Some double-blind, placebo-controlled studies have suggested that magnesium, riboflavin, and feverfew are effective for migraine prophylaxis. However, in a randomized trial of a combination of riboflavin 400 mg, magnesium 300 mg, and feverfew 100 mg versus placebo, both groups evidenced a significant reduction in number of migraines, migraine days, and migraine index. This effect exceeded placebo effects reported in previous migraine studies.13
In the trial, 49 patients received two caplets of the active combination drug or placebo for 3 months. Since a noted effect of riboflavin therapy is orange-colored urine, a small dose of riboflavin (25 mg) was added to the placebo. This dose of riboflavin was thought to have no clinical activity. The primary efficacy endpoint was a 50% or more reduction in migraines.13
The strong response of the placebo group in this trial may be due to the addition of riboflavin to the placebo. Confounding variables such as this yield invalid results. However, individual clinical trials support the potential efficacy of these complementary therapies when used alone, and these agents may possibly be used as adjunctive therapy with established pharmacologic regimens.13
CONCLUSION
The revised guidelines developed by the AAN and the AHS for the use of pharmacologic agents and complementary therapies for migraine prophylaxis provide recommendations that were based on evidence-based clinical trials conducted after the release of the 2000 guidelines. This in-depth analysis gives an oversight of the methods, designs, and results of the clinical trials examining the efficacy of these agents. The updated guidelines address new therapies for the short-term prevention of MAM, namely, frovatriptan, naratriptan, and zolmitriptan. Also, new evidence in correlation with already established trial-based evidence supports the ineffectiveness of lamotrigine for migraine prophylaxis. Finally, the use of complementary therapies, such as herbal formulations, for migraine prophylaxis has been advanced by evidence-based efficacy in clinical trials, providing a new arsenal of evidence-based treatment during a time when the use of herbal formulations has become increasingly popular.
REFERENCES
1. Ashtari F, Shaygannejad V, Akbari M. A double-blind, randomized trial of low-dose topiramate vs propranolol in migraine prophylaxis. Acta Neurol Scand. 2008;118:301-305.
2. Silberstein S, Holland S, Freitag F, et al. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337-1345.
3. Holland S, Silberstein S, Freitag F, et al. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1346-1353.
4. Freitag FG, Collins SD, Carlson HA, et al. A randomized trial of divalproex sodium extended-release tablets in migraine prophylaxis. Neurology. 2002;58:1652-1659.
5. Diener HC, Bussone G, Van Oene JC, et al. Topiramate reduces headache days in chronic migraine: a randomized, double-blind, placebo-controlled study. Cephalalgia. 2007;27:814-823.
6. Diener HC, Hartung E, Chrubasik J, et al. A comparative study of oral acetylsalicylic acid and metoprolol for the prophylactic treatment of migraine. A randomized, controlled, double-blind, parallel group phase III study. Cephalalgia. 2001;21:120-128.
7. Silberstein SD, Elkind AH, Schreiber C, Keywood C. A randomized trial of frovatriptan for the intermittent prevention of menstrual migraine. Neurology. 2004;63:261-269.
8. Bulut S, Berilgen M, Baran A, et al. Venlafaxine versus amitriptyline in the prophylactic treatment of migraine: randomized, double-blind, crossover study. Clin Neurol Neurosurg. 2004;107:44-48.
9. Newman L, Mannix LK, Landy S, et al. Naratriptan as short-term prophylaxis of menstrually associated migraine: a randomized, double-blind, placebo-controlled study. Headache. 2001;41:248-256.
10. Tuchman MM, Hee A, Emeribe U, Silberstein S. Oral zolmitriptan in the short-term prevention of menstrual migraine: a randomized, placebo-controlled study. CNS Drugs. 2008;22:877-886.
11. Gupta P, Singh S, Goyal V, et al. Low-dose topiramate versus lamotrigine in migraine prophylaxis (the Lotolamp study).Headache. 2007;47:402-412.
12. Lipton RB, Göbel H, Einhäupl KM, et al. Petasites hybridus root (butterbur) is an effective preventive treatment for migraine. Neurology. 2004;63:2240-2244.
13. Maizels M, Blumenfeld A, Burchette R. A combination of riboflavin, magnesium, and feverfew for migraine prophylaxis: a randomized trial. Headache. 2004;44:885-890.





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 