[EXCERPTS]
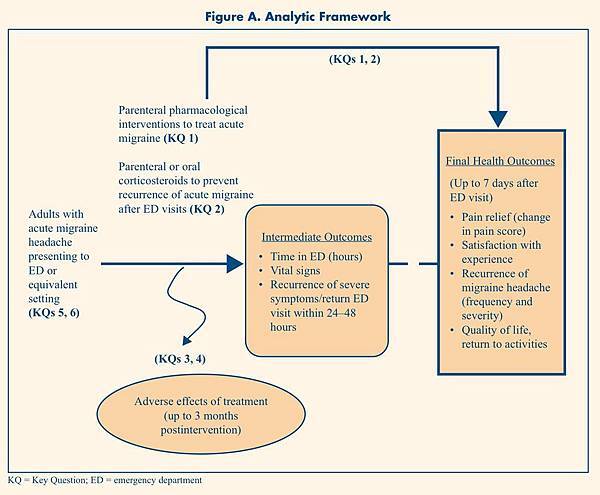
The Key Questions (KQs) are as follows:
- What is the comparative effectiveness of parenteral pharmacological interventions versus standard care, placebo, or an active treatment in the treatment of acute migraine headaches in adults visiting the ED?
- What is the comparative effectiveness of adding parenteral or oral corticosteroids versus adding placebo to acute parenteral pharmacological interventions to prevent recurrence of acute migraine headaches in adults after being treated in the ED?
- What are the associated short-term adverse effects of these parenteral pharmacological interventions, and do they differ across interventions?
- Does the development of adverse events (especially akathisia) differ following the administration of anticholinergic agents and phenothiazines when compared with anticholinergic agents and metoclopramide?
- Do the effectiveness and safety of the parenteral pharmacological interventions vary in different subgroups, including sex, race, duration of headaches, and nonresponders while in the ED?
- Do the effectiveness and safety of adding parenteral or oral corticosteroids to acute parenteral pharmacological interventions vary in different subgroups, including sex, race, duration of headaches, and nonresponders?
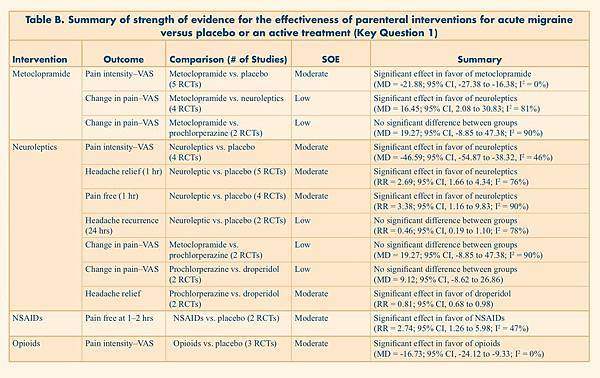
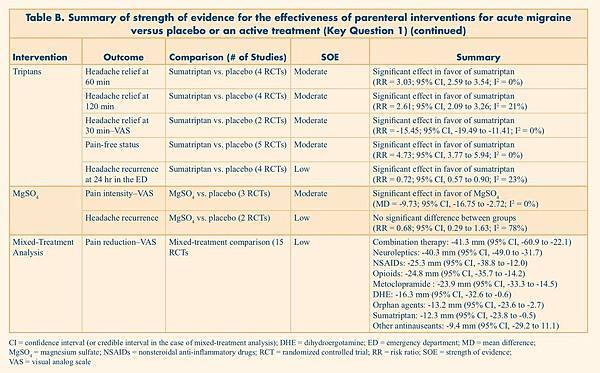
Table B. Summary of strength of evidence for the effectiveness of parenteral interventions for acute migraine versus placebo or an active treatment (Key Question 1)
|
Intervention |
Outcome |
Comparision |
SOE |
Summary |
|
CI = confidence interval (or credible interval in the case of mixed-treatment analysis); DHE = dihydroergotamine; ED = emergency department; MD = mean difference; MgSO4 = magnesium sulfate; NSAIDs = nonsteroidal anti-inflammatory drugs; RCT = randomized controlled trial; RR = risk ratio; SOE = strength of evidence; VAS = visual analog scale |
||||
|
Metoclopramide |
Pain intensity–VAS |
Metoclopramide vs. placebo (5 RCTs) |
Moderate |
Significant effect in favor of metoclopramide (MD = -21.88; 95% CI, -27.38 to -16.38; I2 = 0%) |
|
Change in pain–VAS |
Metoclopramide vs. neuroleptics (4 RCTs) |
Low |
Significant effect in favor of neuroleptics (MD = 16.45; 95% CI, 2.08 to 30.83; I2 = 81%) |
|
|
Change in pain–VAS |
Metoclopramide vs. prochlorperazine |
Low |
No significant difference between groups (MD = 19.27; 95% CI, -8.85 to 47.38; I2 = 90%) |
|
|
Neuroleptics |
Pain intensity–VAS |
Neuroleptics vs. placebo (4 RCTs) |
Moderate |
Significant effect in favor of neuroleptics (MD = -46.59; 95% CI, -54.87 to -38.32, I2 = 46%) |
|
Headache relief |
Neuroleptic vs. placebo (5 RCTs) |
Moderate |
Significant effect in favor of neuroleptics (RR = 2.69; 95% CI, 1.66 to 4.34; I2 = 76%) |
|
|
Pain free (1 hr) |
Neuroleptic vs. placebo (4 RCTs) |
Moderate |
Significant effect in favor of neuroleptics (RR = 3.38; 95% CI, 1.16 to 9.83; I2 = 90%) |
|
|
Headache recurrence (24 hrs) |
Neuroleptic vs. placebo (2 RCTs) |
Low |
No significant difference between groups (RR = 0.46; 95% CI, 0.19 to 1.10; I2 = 78%) |
|
|
Change in pain–VAS |
Metoclopramide vs. prochlorperazine |
Low |
No significant difference between groups (MD = 19.27; 95% CI, -8.85 to 47.38; I2 = 90%) |
|
|
Change in pain–VAS |
Prochlorperazine vs. droperidol (2 RCTs) |
Low |
No significant difference between groups (MD = 9.12; 95% CI, -8.62 to 26.86) |
|
|
Headache relief |
Prochlorperazine vs. droperidol (2 RCTs) |
Moderate |
Significant effect in favor of droperidol (RR = 0.81; 95% CI, 0.68 to 0.98) |
|
|
NSAIDs |
Pain free at 1–2 hrs |
NSAIDs vs. placebo |
Moderate |
Significant effect in favor of NSAIDs (RR = 2.74; 95% CI, 1.26 to 5.98; I2 = 47%) |
|
Opioids |
Pain intensity–VAS |
Opioids vs. placebo (3 RCTs) |
Moderate |
Significant effect in favor of opioids (MD = -16.73; 95% CI, -24.12 to -9.33; I2 = 0%) |
|
Triptans |
Headache relief at 60 min |
Sumatriptan vs. placebo (4 RCTs) |
Moderate |
Significant effect in favor of sumatriptan (RR = 3.03; 95% CI, 2.59 to 3.54; I2 = 0%) |
|
Headache relief at 120 min |
Sumatriptan vs. placebo (4 RCTs) |
Moderate |
Significant effect in favor of sumatriptan (RR = 2.61; 95% CI, 2.09 to 3.26; I2 = 21%) |
|
|
Headache relief at 30 min–VAS |
Sumatriptan vs. placebo (2 RCTs) |
Moderate |
Significant effect in favor of sumatriptan (RR = -15.45; 95% CI, -19.49 to -11.41; I2 = 0%) |
|
|
Pain-free status |
Sumatriptan vs. placebo (5 RCTs) |
Moderate |
Significant effect in favor of sumatriptan (RR = 4.73; 95% CI, 3.77 to 5.94; I2 = 0%) |
|
|
Headache recurrence at 24 hr in the ED |
Sumatriptan vs. placebo (4 RCTs) |
Low |
Significant effect in favor of sumatriptan (RR = 0.72; 95% CI, 0.57 to 0.90; I2 = 23%) |
|
|
MgSO4 |
Pain intensity–VAS |
MgSO4 vs. placebo |
Moderate |
Significant effect in favor of MgSO4 (MD = -9.73; 95% CI, -16.75 to -2.72; I2 = 0%) |
|
Headache recurrence |
MgSO4 vs. placebo |
Low |
No significant difference between groups (RR = 0.68; 95% CI, 0.29 to 1.63; I2 = 78%) |
|
|
Mixed-Treatment Analysis |
Pain reduction–VAS |
Mixed-treatment comparison (15 RCTs) |
Low |
Combination therapy: -41.3 mm (95% CI, -60.9 to -22.1) Neuroleptics: -40.3 mm (95% CI, -49.0 to -31.7) NSAIDs: -25.3 mm (95% CI, -38.8 to -12.0) Opioids: -24.8 mm (95% CI, -35.7 to -14.2) Metoclopramide : -23.9 mm (95% CI, -33.3 to -14.5) DHE: -16.3 mm (95% CI, -32.6 to -0.6) Orphan agents: -13.2 mm (95% CI, -23.6 to -2.7) Sumatriptan: -12.3 mm (95% CI, -23.8 to -0.5) Other antinauseants: -9.4 mm (95% CI, -29.2 to 11.1) |
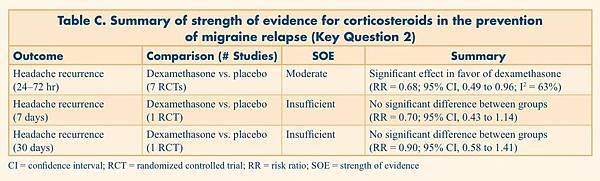
Table C. Summary of strength of evidence for corticosteroids in the prevention of migraine relapse (Key Question 2)
|
Outcome |
Comparison (# Studies) |
SOE |
Summary |
|
CI = confidence interval; RCT = randomized controlled trial; RR = risk ratio; SOE = strength of evidence |
|||
|
Headache recurrence (24–72 hr) |
Dexamethasone vs. placebo (7 RCTs) |
Moderate |
Significant effect in favor of dexamethasone (RR = 0.68; 95% CI, 0.49 to 0.96; I2 = 63%) |
|
Headache recurrence |
Dexamethasone vs. placebo (1 RCT) |
Insufficient |
No significant difference between groups (RR = 0.70; 95% CI, 0.43 to 1.14) |
|
Headache recurrence |
Dexamethasone vs. placebo (1 RCT) |
Insufficient |
No significant difference between groups (RR = 0.90; 95% CI, 0.58 to 1.41) |
Table D. Summary of strength of evidence for the development of akathisia with the addition of anticholinergics to metoclopramide and phenothiazine (Key Question 4)
|
Outcome |
Comparison (# Studies) |
SOE |
Summary |
|
CI = confidence interval; OR = odds ratio; RCT = randomized controlled trial; SOE = strength of evidence |
|||
|
Akathisia |
Metoclopramide + anticholinergic vs. phenothiazine + anticholinergic (1 RCT) |
Insufficient |
No significant difference between groups (OR = 1.50; 95% CI, 0.24 to 9.52) |
|
Prochlorperazine + diphenhydramine vs. prochlorperazine (1 RCT) |
Insufficient |
No significant difference (OR = 0.46; 95% CI, 0.17 to 1.28) |
|
Conclusions
This report provides the most comprehensive synthesis to date of the comparative effectiveness of parenteral pharmacological interventions versus standard care, placebo, or an active treatment in the management of acute migraine headaches in adults presenting to the ED or an equivalent setting. Overall, there are several important conclusions from this work. First, many agents appear to be effective in the treatment of acute migraine headache when compared with placebo. Neuroleptic monotherapy and DHE in combination with either metoclopramide or neuroleptics appear to be the most effective options for pain relief (VAS). Second, several treatments reported here provide insufficient evidence for continued use (e.g., lidocaine, antihistamines, sodium valproate). Third, systemic corticosteroids effectively prevent relapses, especially in patients with prolonged headaches. Finally, the list of adverse effects is extensive, albeit they vary among agents and classes of drugs. Overall, the effectiveness of therapies described here must be weighed against their side effects to derive a strategy for treating patients with this common disorder. While the evidence collated here is an important step, more research is required in order to identify the most effective and safest parenteral medication for acute migraine.
[Link to free full-text Comparative Effectiveness Review PDF]






 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 