(圖片來自http://www.rvc.ac.uk)
資料來源:
Emergency Diagnostic And Therapeutic Procedures
PHARMACOLOGIC MEANS TO ANALGESIA: MAJOR ANALGESICS
OPIOIDS
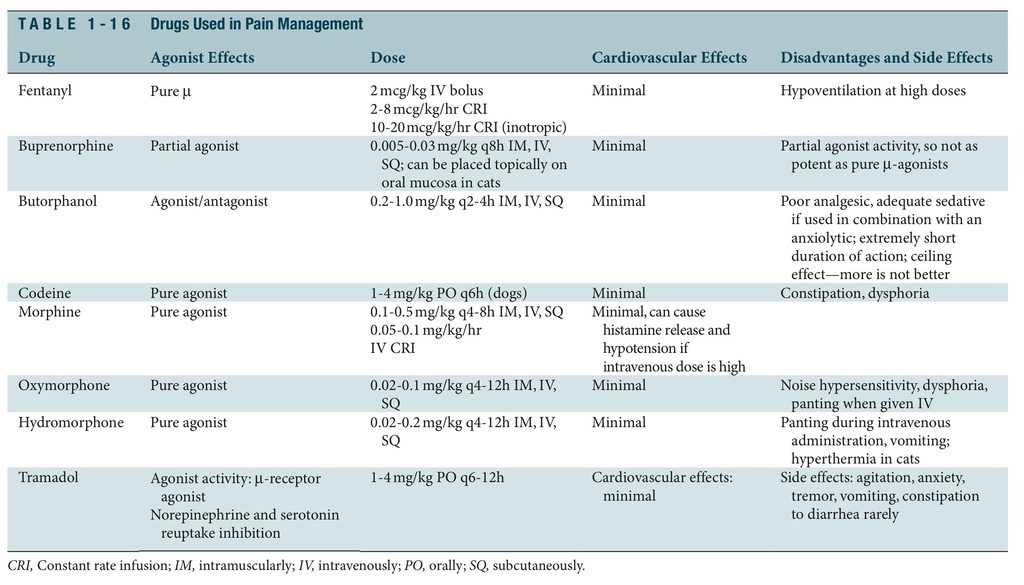
An opioid is any natural or synthetic drug that is derived from the poppy, which interacts with opiate receptors identified on cell membranes. The drugs from this class constitute the most effective means of controlling acute, perioperative, and chronic pain in human and veterinary medicine (Table 1-16). Their physiologic effects result from the interaction with one or more of at least five endogenous opioid receptors (, , , , and ). -Receptor ago- nists are noted for their ability to produce profound analgesia with mild sedation. These drugs diminish “windup,” the hyperexcitable state resulting from an afferent volley of noci- ceptive impulses. They elevate the pain threshold and are used preemptively to prevent acute pain.
As a class, opioids cause CNS depression with their intense analgesia. Dose-related respi- ratory depression reflects diminished response to carbon dioxide levels. Cardiac depression is secondary only to bradycardia and is more likely with certain opioids such as morphine and oxymorphone. Narcotics produce few if any clinically significant cardiovascular effects in dogs and cats; they are considered cardiac soothing or sparing. Because opioids increase intracranial and intraocular pressure, use them more cautiously in patients with severe cra- nial trauma and or ocular lesions. Opioids directly stimulate the chemoreceptor trigger zone and may cause nausea and vomiting. Most opioids depress the cough reflex via a central mechanism; this may be helpful in patients recovering from endotracheal intubation irri- tation. A key characteristic of opioids that makes them desirable for use in emergency and critical care situations is their reversibility. Antagonists block or reverse the effect of agonists by combining with receptors and producing minimal or no effects. Administer all reversal agents, such as naloxone and naltrexone, slowly if given intravenously and to effect.
a2-AGONISTS
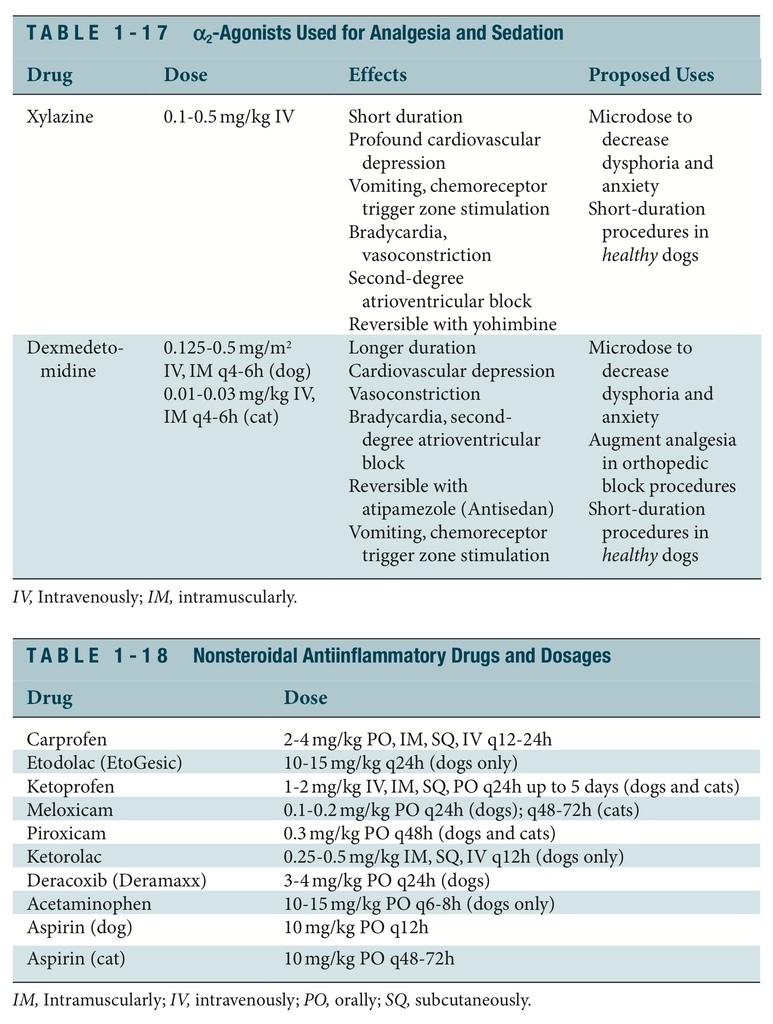
As a class of drugs, 2-agonists warrant special attention because most members of the group possess potent analgesic power at doses that are capable of causing sedation, CNS depression, cardiovascular depression, and even general anesthetic states. Originally devel- oped for antihypertensive use, 2-agonists quickly have attained sedative analgesic status in veterinary medicine (Table 1-17). Like the opioids, 2-agonists produce their effects by aggravating -adrenergic receptors in the CNS and periphery.
NONSTEROIDAL ANTIINFLAMMATORY DRUGS
NSAIDs, which classically have been used to treat chronic pain and inflammation, as well as cardiovascular diseases, have taken on a new role in the treatment of perioperative and acute pain. Recently developed potent oral and parenteral forms of these drugs have compared favorably with and sometimes superiorly to opioids for treatment of acute inflammation and pain (Table 1-18). Nonsteroidal drugs can be used alone, but their best use is that of providing synergistic analgesia with different classes of analgesics (narcotics) or modalities (local, regional, and epidural analgesia, physical therapy, acupuncture).
Most NSAIDS act by inhibition of cyclooxygenase (COX; also known as prostaglandin synthetase), an enzyme that catalyzes the incorporation of molecular oxygen into arachi- donic acid to produce mediators of inflammation. There are at least a few forms of COX, among them COX-1, the major constitutive enzyme primarily involved in normal phys- iologic functions, and COX-2, the enzyme responsible for most of the hyperalgesia and pain responses experienced after tissue injury or trauma. Some NSAIDS inhibit COX and lipoxygenase activity. Most of the currently available oral and parenteral NSAIDS for small animal medicine and surgery target the COX pathways predominantly, although one (tep- oxalin) is thought to inhibit both pathways. Inhibition of COX-1 and COX-2 can inhibit the protective effects and impair platelet aggregation and lead to gastrointestinal ulceration.
There are definite contraindications and relative contraindications for the use of NSAIDs. NSAIDs should not be administered to patients with renal or hepatic insufficiency, dehydration, hypotension, or conditions that are associated with low circulating volume (CHF, unregulated anesthesia, shock), or evidence of ulcerative gastrointestinal disease. Trauma patients should be stabilized completely regarding vascular volume, tone, and pressure before the use of NSAIDs. Patients receiving concurrent administration of other NSAIDs or corticosteroids, or those considered to be cushingoid, should be evaluated care- fully for an adequate “washout” period (time of clearance of drug from the system) before use of an NSAID or before switching NSAIDs. Patients with coagulopathies, particularly those that are caused by platelet number or function defects or those caused by factor deficiencies, and patients with severe, uncontrolled asthma or other bronchial disease are probably not the patients in which to use NSAIDs. Other advice is that NSAIDs not be administered to pregnant patients or to females attempting to become pregnant because COX-2 induction is necessary for ovulation and subsequent implantation of the embryo. The administration of NSAIDs should be considered only in the well-hydrated, normoten- sive dog or cat with normal renal or hepatic function, with no hemostatic abnormalities and no concurrent steroid administration.
NSAIDs can be used in many settings of acute and chronic pain and inflammation. Among these are well-stabilized musculoskeletal trauma and surgical pain, osteoarthri- tis management, meningitis, mastitis, animal bite and other wound healing, mammary or transitional cell carcinoma, epithelial (dental, oral, urethral) inflammation, ophthalmologic procedures, and dermatologic or otic disease. Whereas opioids seem to have an immediate analgesic effect when administered, most NSAIDS will take up to 30 minutes for their effect to be recognized. Therefore most perioperative or acute NSAID use is part of a balanced pain management scheme, one that uses narcotics and local anesthetic techniques. NSAIDs are devoid of many of the side effects of narcotic administration—namely, decreased gastrointestinal motility, altered sensorium, nausea and vomiting, and sedation. NSAIDs are also devoid of many of the side effects of steroid administration—namely, suppression of the pituitary adrenal axis.
Nonsteroidal Antiinflammatory Drugs in Cats
The toxic effects of salicylates in cats are well documented. Cats are susceptible because of slow clearance and dose-dependent elimination caused by deficient glucuronidation in this species. Because of this, the dose and the administration interval of most commonly used NSAIDs need to be altered in order for these drugs to be used. Cats that have been given canine doses of NSAIDs (twice daily or even once daily repetitively) may show hyper- thermia, hemorrhagic or ulcerative gastritis, kidney and liver injury, hyperthermia, respiratory alkalosis, and metabolic acidosis. Acute and chronic toxicities of NSAIDs have been reported in cats, especially after repeat once-daily administration. Ketoprofen, flunixin, aspirin, carprofen, and meloxicam have been administered safely to cats, although like most antibiotics and other medications, they are not approved and licensed for use in cats. An important note, though, is that administration intervals ranging from 48 to 96 hours have been used, and antithrombotic effects often can be achieved at much lower doses than those required to treat fevers and inflammation. I recommend the use of no loading doses, minimum 48-hour administration intervals, and assurance of adequate circulating blood volume, BP, and renal function.
Because many of the NSAIDs are used off-label in cats, it is imperative that the clini- cian carefully calculate the dose, modify the administration interval, and communicate this information to the client before dispensing the drug. Even drugs that come in liquid form (meloxicam), if administered to cats via box-labeled directions used for dogs, will be given in near toxic doses. To worsen the misunderstanding about dosages for cats, drops from man- ufacturer's bottles often are calibrated drops; when these same liquids are transferred into pharmacy syringes for drop administration, the calibration, of course, is lost, and the animal potentially receives an overdose. A more accurate method of dispensing oral NSAIDs for use in cats and administering them is to calculate the dose in milligrams and determine the exact number of milliliters to administer, rather than using the drop method.
ADJUNCTIVE ANALGESIC DRUGS
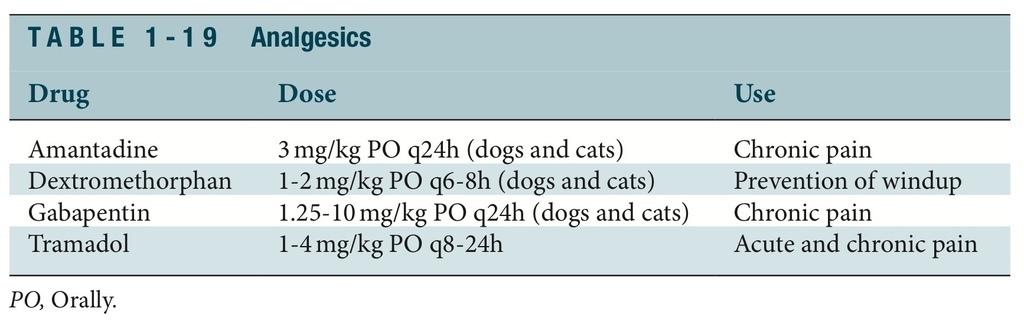
Local anesthetic agents are the major class of drugs used as peripheral-acting analge- sics (Table 1-19). Local anesthetics block the transmission of pain impulses at the peripheral nerve nociceptor regions. Local anesthetics may be used to block peripheral nerves or inhibit nerve “zones” through use of regional techniques. Although all local anesthetics are capable of providing pain relief, agents with a longer duration of action are preferred for pain management purposes. Bupivacaine is an example of a long- acting local anesthetic drug that is used along with lidocaine for long-acting pain relief. A single dose of bupivacaine injected at a local site will provide local anesthesia and analgesia for 6 to 10 hours.
ANALGESIA: MINOR ANALGESICS
KETAMINE
Ketamine classically was considered a dissociative anesthetic, but it also has potent activity as an N-methyl-D-aspartate (NMDA) receptor antagonist. This receptor, located in the CNS, mediates windup and central sensitization (a pathway from acute to chronic pain). Blockade of this receptor with microdoses of ketamine results in the ability to provide body surface, somatic, and skin analgesia with potentially lower doses of opioids and -agonists. Loading doses of 0.5 to 2 mg/kg are used IV with CRIs of 2 to 20 mcg/kg/min. In and of itself, this drug possesses little to no analgesic ability and indeed in high doses alone often can aggravate, sensitize, or excite the animal in subacute or acute pain.
AMANTADINE
Amantadine is another NMDA blocker that has been used for its antiviral properties and stabilizing effects in Parkinson disease. Amantadine has been used for neuropathic pain in human beings but is available only in an oral form. Suggested starting doses for cats and dogs range from 3 to 5 mg/kg orally (PO) daily. When the drug is given PO or IV, patients are unlikely to develop behavioral or cardiorespiratory effects with ketamine or amantadine.
TRAMADOL
Tramadol is an analgesic that possesses weak opioid -agonist activity and norepinephrine and serotonin reuptake inhibition. Tramadol is useful for mild to moderate pain in small animals. Although the parent compound has very weak opioid activity, the metabolites have excellent binding affinity for the receptor. Tramadol has been used for perisurgical pain control when given PO in cats and dogs at a dose of 1 to 4 mg/kg PO once to four times daily. Regardless of its affinity for the opioid receptors, the true mechanism of action of tra- madol in companion animals remains largely unknown.
GABAPENTIN
Gabapentin is a synthetic analog of -aminobutyric acid (GABA). Originally introduced as an antiepileptic drug, the mechanism of action of gabapentin remains somewhat unclear in veterinary medicine. The drug is among a number of commonly used antiepi- leptic medications used to treat central pain in human beings. The rationale for use is the ability of the drugs to suppress discharge in pathologically altered neurons. Gabapentin does this through calcium channel modulation without binding to glutamate receptors. Chronic, burning, neuropathic, and lancinating pain in small animals responds well to 1 to 10 mg/kg PO daily.
Lidocaine administered as an intravenous CRI (50 to 75 mcg/kg/min in dogs, 1 to 10 mcg/ kg/min in cats) is effective in the treatment of chronic neuropathic pain and periosteal and peritoneal pain (e.g., pancreatitis). Mexiletine, an oral sodium channel blocker, can be used as an alternative to injectable lidocaine for provision of background analgesia.
ANXIOLYTICS AND SEDATIVES
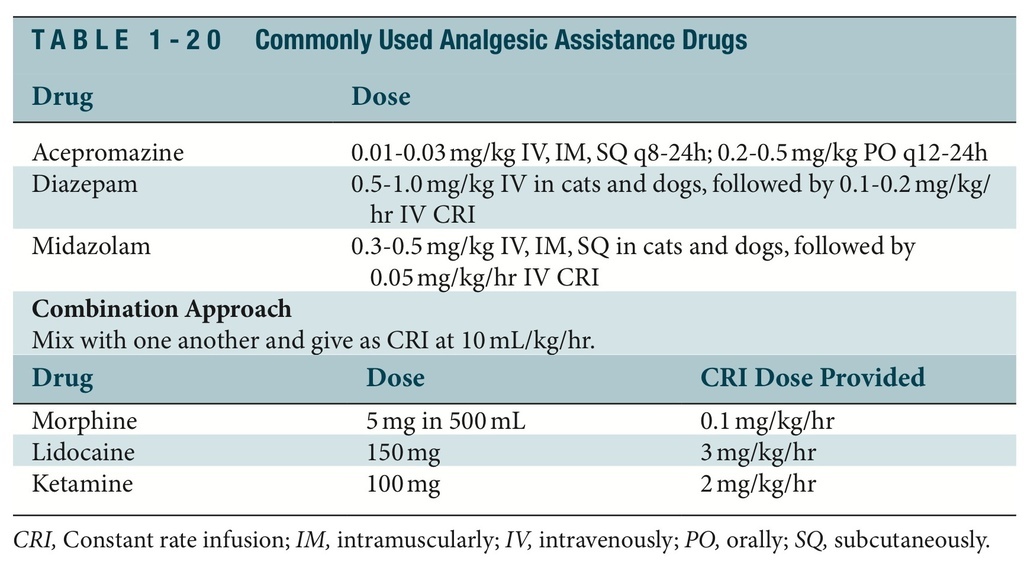
Many drugs (Table 1-20) are used in combination with opioids, 2-agonists, and ketamine to provide anxiolysis and sedation.
LOCAL AND REGIONAL TECHNIQUES FOR THE EMERGENT PATIENT
Injection of local anesthetic solution into the connective tissue surrounding a particular nerve produces loss of sensation (sensory blockade) and/or paralysis (motor nerve blockade) in the region supplied by the nerve. Local anesthetics also may be administered epidurally, intratho- racically, intraperitoneally, and intraarticularly. Lidocaine and bupivacaine are the most com- monly administered local anesthetics. Lidocaine provides for quick, short-acting sensory and motor impairment. Bupivacaine provides for later-onset, longer-lasting desensitization with- out motor impairment. Combinations of the two agents diluted with saline are used frequently to provide for quick-onset analgesia that lasts 4 to 6 hours in most patients. Adding a narcotic and/or an 2 agent often maximizes the analgesia and increases the pain-free interval to 8 to 18 hours. Epinephrine-free and preservative-free solutions are recommended. Precision placement of anesthetic close to nerves, roots, or plexuses is improved with the use of a stimu- lating nerve locator. Cats seem to be more sensitive to the effects of local anesthetics; therefore the lower ends of most dose ranges are used for blockades in this species.
Unlike most instances of general anesthesia, during which the animal is rendered uncon- scious and nerve transmission is decreased by virtue of CNS depression, local and regional techniques block the initiation of noxious signals, thereby effectively preventing pain from entering the CNS. This is an effective means not only of preventing initial pain but also of reducing the changes that take place in the dorsal horn of the spinal cord, spinothalamic tracts, limbic and reticular activating centers, and cortex. Frequently the neurohormonal response that is stimulated in pain and stress is blunted as well. Overall, the patient has fewer local and systemic adverse effects of pain, disease processes are minimized, chronic pain states are unlikely, and outcome is improved. Regional techniques are best used as part of an analgesic regimen that consists of their continuous administration, narcotics, -agonists, anxiolytics, and good nursing.
TOPICAL AND INFILTRATIVE BLOCKADE
Lidocaine can be added to sterile lubricant in a one-to-one concentration to provide decreased sensation for urinary catheterization, nasal catheter insertion, minor road burn analgesia, and pyotraumatic dermatitis analgesia. Proparacaine is a topical anesthetic useful for corneal or scleral injuries. Local anesthetics can be used to infiltrate areas of damage or surgery through use of long-term continuous drainage catheters and small, portable infu- sion pumps. This is an effective means of providing days of analgesia for massive surgical or traumatic soft tissue injury. Even without the catheter, incisional or regional soft tissue blocking using a combination of 1 to 2mg of lidocaine per kilogram and 0.5 to 2mg of bupivacaine per kilogram diluted with equal volume of saline and 1:9 with sodium bicarbonate is effective for infiltrating large areas of injury.
CRANIAL NERVE BLOCKADE
Administration of local anesthetic drugs around the infraorbital, maxillary, ophthalmic mental, and alveolar nerves can provide excellent analgesia for dental, orofacial, and oph- thalmic trauma and surgical procedures. Each nerve may be desensitized by injecting 0.1 to 0.3 mL of a 2% lidocaine hydrochloride solution and 0.1 to 0.3 mL of a 0.5% bupivacaine solution using a 1.2 to 2.5-cm, 22- to 25-gauge needle. Precise placement perineurally versus intraneurally (neuroma formation common) is enhanced by using catheters in the foramen versus needle administration. Always perform aspiration before administration to rule out intravascular injection of agents.
INTRAPLEURAL BLOCKADE
Intrapleural blockade is used to provide analgesia for thoracic, lower cervical, cranial abdominal, and diaphragmatic pain. After aseptic preparation, place a small through-the- needle (20 to 22-gauge) catheter in the thoracic cavity between the seventh and ninth inter- costal space on the midlateral aspect of the thorax. Aseptically mix a 0.5- to 1-mg/kg lidocaine and a 0.2 to 0.5-mg/kg bupivacaine dose with volume of saline equal to the vol- ume of bupivacaine, and slowly inject it over a period of 2 to 5 minutes after aspiration to ensure that no intravascular injection occurs. Depending on where the lesion is, position the patient to allow the intrapleural infusion to “coat” the area. Most effective is positioning the patient in dorsal recumbency for several minutes after the block to make sure local anesthetic occupies the paravertebral gutters and hence the spinal nerve roots. The block should be repeated every 3 hours in dogs and every 8 to 12 hours in cats. Secure the catheter to the skin surface for repetitive administration.
BRACHIAL PLEXUS BLOCKADE
Administration of local anesthetic around the brachial plexus provides excellent analgesia for forelimb surgery, particularly distal to the shoulder, and amputations. Nerve locator– guided techniques are much more accurate and successful than blind placement of local anesthetic; however, even the latter is useful.
To administer a brachial plexus blockade, follow this procedure:
1. Aseptically prepare a small area of skin over the point of the shoulder.
2. Insert a 22-gauge, 11⁄2- to 3-inch spinal needle medial to the shoulder joint, axial to
the lesser tubercle, and advance it caudally, medial to the body of the scapula, and toward the costochondral junction of the first rib. Aspirate first before injection to make sure that intravenous injection does not occur.
3. Inject one third of the volume of local anesthetic mix, and then slowly withdraw the needle and fan dorsally and ventrally while infusing the remaining fluid.
4. Local anesthetic doses are similar to those for intrapleural blockade.
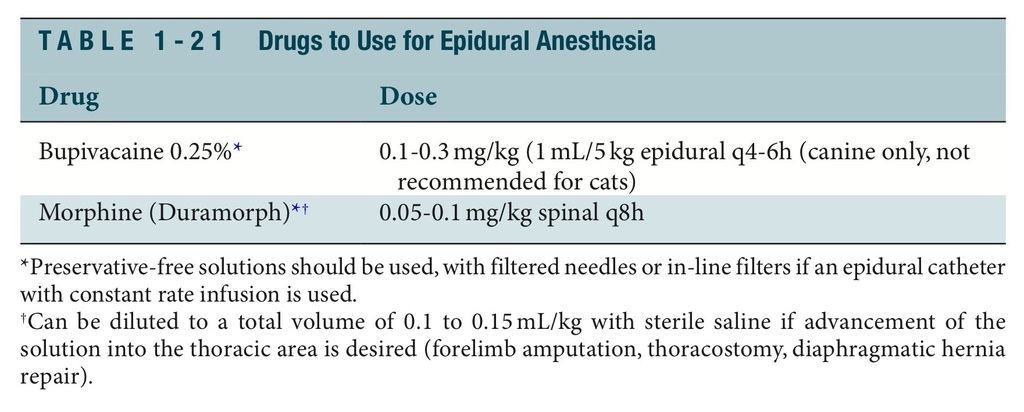
EPIDURAL ANESTHESIA AND ANALGESIA
Epidural analgesia refers to the injection of an opioid, a phencyclidine, an -agonist, or an NSAID into the epidural space. Epidural anesthesia refers to the injection of a local anes- thetic. In most patients a combination of the two is used. Epidural analgesia and anesthe- sia are used for acute and chronic surgical pain or traumatically induced pain in the pelvis, tail, perineum, hind limbs, abdomen, and thorax (Table 1-21). Procedures in which epidural analgesia and anesthesia are useful include forelimb and hindlimb amputation, tail or perineal procedures, cesarean sections, diaphragmatic hernia repair, pancreatitis, peritonitis, and intervertebral disk disease. Epidural blocks performed using opioids or bupivacaine will not result in hindlimb paresis or decreased urinary or anal tone (incon- tinence), unlike lidocaine or mepivacaine epidural blocks. Morphine is one of the most useful opioids for administration in the epidural space because of its slow systemic absorption. Epidural catheters used for the instillation of drugs through CRI or intermit- tent injection can be placed in dogs and cats. Routinely placed at the lumbosacral junc- tion, these catheters are used with cocktails including preservative-free morphine, bupivacaine, dexmedetomidine, and ketamine. Extremely effective for preventing windup pain in the peritoneal cavity or caudal half of the body, the catheters may be maintained if placed aseptically for 7 to 14 days.
To provide epidural analgesia or anesthesia, follow this procedure:
1. Position the animal in lateral or sternal recumbency.
2. Clip and aseptically scrub over the lumbosacral site.
3. Palpate the craniodorsal-most extent of the wings of the ileum bilaterally and draw an
imaginary line through them to envision the spine of L7, located immediately behind
the imaginary line.
4. Advance a 20- to 22-gauge, 11⁄2- to 3-inch spinal or epidural needle through the skin
just caudal to the spine of L7.
5. The needle will lose resistance as it is introduced into the epidural space. Drop saline
into the hub of the needle, and the saline will be pulled into the epidural space as the needle enters.
INTERCOSTAL NERVE BLOCKS
Discrete intercostal nerve blocks can provide effective analgesia for traumatic or postsurgi- cal pain. Identify the area of the injury, and infiltrate three segments on either side of the injury with analgesic.
To perform an intercostal nerve block, follow this procedure:
1. Clip and aseptically scrub the dorsal and ventral third of the chest wall.
2. Palpate the intercostal space as far dorsally as possible.
3. Use a 25-gauge, 5⁄8-inch needle at the caudolateral aspect of the affected rib segments
and those cranial and caudal.
4. Direct the tip of the needle caudally such that the tip of the needle “drops” off of
the caudal rib. (This places the needle tip in proximity to the neuromuscular bundle that contains the intercostal nerve that runs in a groove on the caudome- dial surface of the rib.)
5. Aspirate to confirm that the drug will not go IV.
6. Inject while slowly withdrawing the needle. Inject 0.5 to 1.0 mL at each site, depend-
ing on the size of the animal.
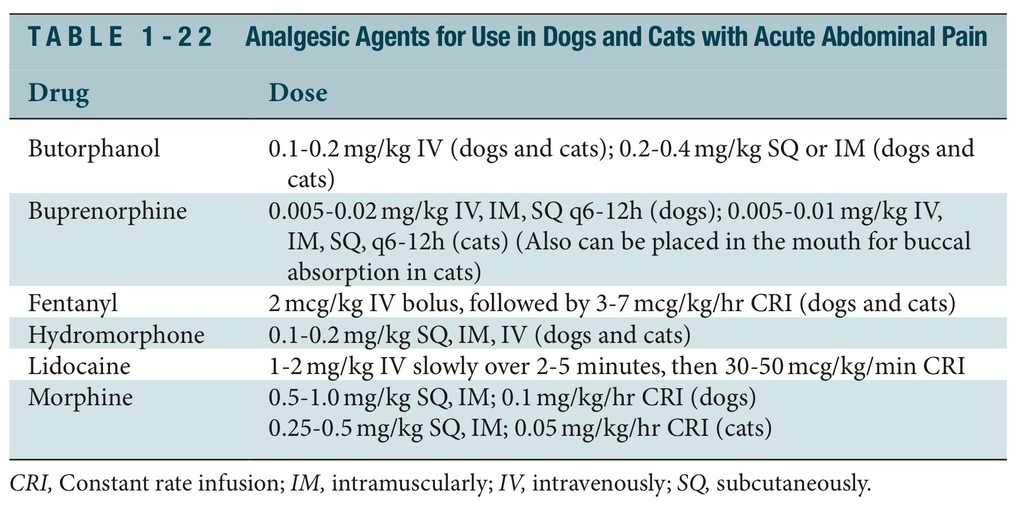
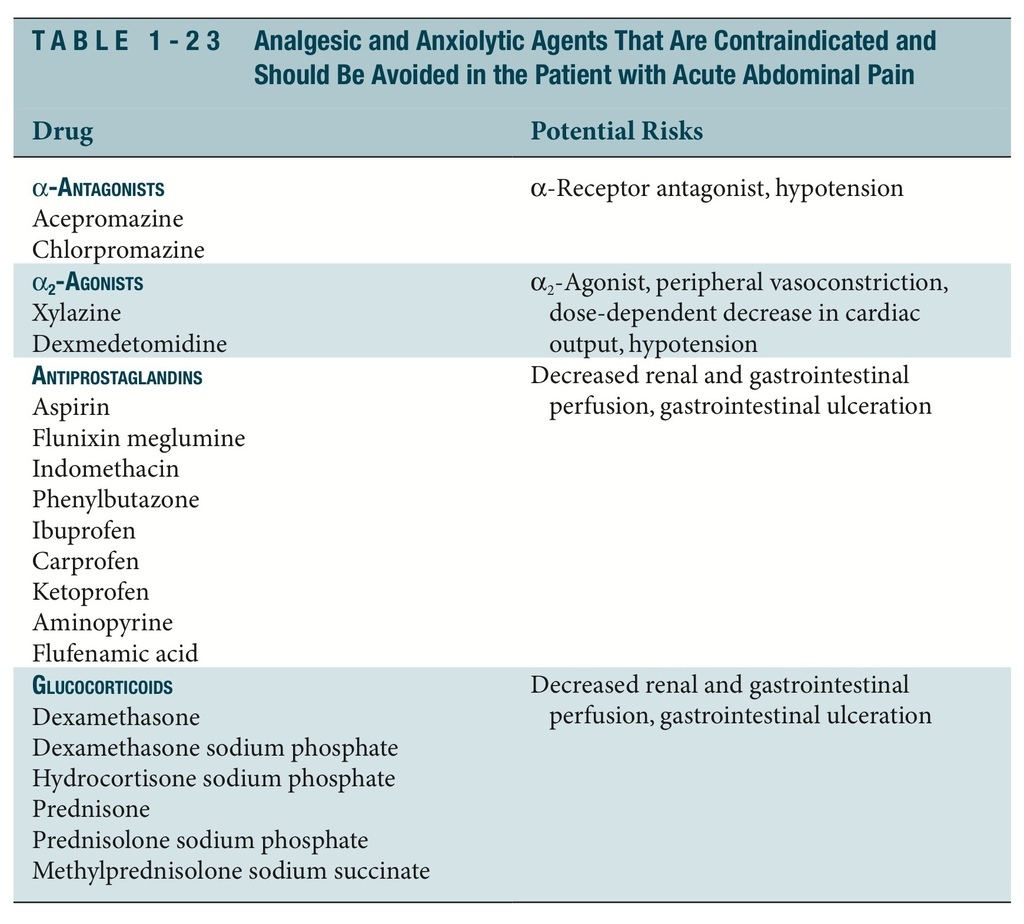
ANALGESIA
The administration of analgesic agents to any patient with acute abdominal pain is one of the most important therapies in the initial stages of case management. Table 1-22 lists analgesic drugs for use in the patient with an acute condition in abdomen. Table 1-23 lists anal- gesic and anxiolytic drugs to avoid in the patient with an acute condition in abdomen.
Additional Reading
Gaynor JS, Muir WW: Handbook of veterinary pain management, St Louis, 2003, Mosby. Melzack R, Wall PD: Handbook of pain management: a clinical companion to Wall and Melzack's
textbook of pain, Churchill Livingstone, 2003, Edinburgh.
Muir WW, Hubbell JAE, Skarda RT, et al: Handbook of veterinary anesthesia, ed 3, St Louis, 2000,
Mosby.






 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 