Information sourced from The Cochrane Library:
Topical antifungals for seborrhoeic dermatitis
Abstract
Background
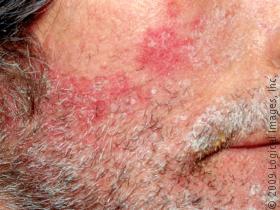
Seborrhoeic dermatitis is a chronic inflammatory skin condition that is distributed worldwide. It commonly affects the scalp, face and flexures of the body. Treatment options include antifungal drugs, steroids, calcineurin inhibitors, keratolytic agents and phototherapy.
Objectives
To assess the effects of antifungal agents for seborrhoeic dermatitis of the face and scalp in adolescents and adults.
A secondary objective is to assess whether the same interventions are effective in the management of seborrhoeic dermatitis in patients with HIV/AIDS.
Search methods
We searched the following databases up to December 2014: the Cochrane Skin Group Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL) (2014, Issue 11), MEDLINE (from 1946), EMBASE (from 1974) and Latin American Caribbean Health Sciences Literature (LILACS) (from 1982). We also searched trials registries and checked the bibliographies of published studies for further trials.
Selection criteria
Randomised controlled trials of topical antifungals used for treatment of seborrhoeic dermatitis in adolescents and adults, with primary outcome measures of complete clearance of symptoms and improved quality of life.
Data collection and analysis
Review author pairs independently assessed eligibility for inclusion, extracted study data and assessed risk of bias of included studies. We performed fixed-effect meta-analysis for studies with low statistical heterogeneity and used a random-effects model when heterogeneity was high.
Main results
We included 51 studies with 9052 participants. Of these, 45 trials assessed treatment outcomes at five weeks or less after commencement of treatment, and six trials assessed outcomes over a longer time frame. We believe that 24 trials had some form of conflict of interest, such as funding by pharmaceutical companies.
Among the included studies were 12 ketoconazole trials (N = 3253), 11 ciclopirox trials (N = 3029), two lithium trials (N = 141), two bifonazole trials (N = 136) and one clotrimazole trial (N = 126) that compared the effectiveness of these treatments versus placebo or vehicle. Nine ketoconazole trials (N = 632) and one miconazole trial (N = 47) compared these treatments versus steroids. Fourteen studies (N = 1541) compared one antifungal versus another or compared different doses or schedules of administration of the same agent versus one another.
Ketoconazole
Topical ketoconazole 2% treatment showed a 31% lower risk of failed clearance of rashes compared with placebo (risk ratio (RR) 0.69, 95% confidence interval (CI) 0.59 to 0.81, eight studies, low-quality evidence) at four weeks of follow-up, but the effect on side effects was uncertain because evidence was of very low quality (RR 0.97, 95% CI 0.58 to 1.64, six studies); heterogeneity between studies was substantial (I2 = 74%). The median proportion of those who did not have clearance in the placebo groups was 69%.
Ketoconazole treatment resulted in a remission rate similar to that of steroids (RR 1.17, 95% CI 0.95 to 1.44, six studies, low-quality evidence), but occurrence of side effects was 44% lower in the ketoconazole group than in the steroid group (RR 0.56, 95% CI 0.32 to 0.96, eight studies, moderate-quality evidence).
Ketoconozale yielded a similar remission failure rate as ciclopirox (RR 1.09, 95% CI 0.95 to 1.26, three studies, low-quality evidence). Most comparisons between ketoconazole and other antifungals were based on single studies that showed comparability of treatment effects.
Ciclopirox
Ciclopirox 1% led to a lower failed remission rate than placebo at four weeks of follow-up (RR 0.79, 95% CI 0.67 to 0.94, eight studies, moderate-quality evidence) with similar rates of side effects (RR 0.9, 95% CI 0.72 to 1.11, four studies, moderate-quality evidence).
Other antifungals
Clotrimazole and miconazole efficacies were comparable with those of steroids on short-term assessment in single studies.
Treatment effects on individual symptoms were less clear and were inconsistent, possibly because of difficulties encountered in measuring these symptoms.
Evidence was insufficient to conclude that dose or mode of delivery influenced treatment outcome. Only one study reported on treatment compliance. No study assessed quality of life. One study assessed the maximum rash-free period but provided insufficient data for analysis. One small study in patients with HIV compared the effect of lithium versus placebo on seborrhoeic dermatitis of the face, but treatment outcomes were similar.
Authors' conclusions
Ketoconazole and ciclopirox are more effective than placebo, but limited evidence suggests that either of these agents is more effective than any other agent within the same class. Very few studies have assessed symptom clearance for longer periods than four weeks. Ketoconazole produced findings similar to those of steroids, but side effects were fewer. Treatment effect on overall quality of life remains unknown. Better outcome measures, studies of better quality and better reporting are all needed to improve the evidence base for antifungals for seborrhoeic dermatitis.
Okokon EO, Verbeek JH, Ruotsalainen JH, Ojo OA, Bakhoya VN. Topical antifungals for seborrhoeic dermatitis. Cochrane Database of Systematic Reviews 2015, Issue 5. Art. No.: CD008138. DOI: 10.1002/14651858.CD008138.pub3.
[Free full-text PDF | Cochrane Library abstract]





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 